| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
PDE5/phosphodiesterase 5
When 1 μM sildenafil was pretreated instead of serotonin stimulation alone, it improved phosphorylation of ERK1/ERK2, increased the percentage of cells in S phase, and promoted cell proliferation (P<0.05). The OD value increased dramatically to 0.33 after pretreatment with 1 μM sildenafil citrate and serotonin stimulation, which was significantly different from serotonin stimulation alone (P<0.05). The phosphorylation of ERK1/ERK2 elicited by serotonin was considerably increased by 1 μM sildenafil [2]. |
|---|---|
| 体外研究 (In Vitro) |
当1 μM西地那非预处理代替单独的5-羟色胺刺激时,可以改善ERK1/ERK2的磷酸化,增加S期细胞的百分比,促进细胞增殖(P<0.05)。 1 μM枸橼酸西地那非预处理和5-羟色胺刺激后,OD值急剧增加至0.33,与单独5-羟色胺刺激有显着差异(P<0.05)。 1 μM 西地那非显着增加了血清素引起的 ERK1/ERK2 磷酸化 [2]。
西地那非(1 μmol/L)增强血清素(10 μmol/L)诱导的猪肺动脉平滑肌细胞(PASMCs)增殖,表现为MTT检测吸光度值增加、S期细胞比例升高、增殖细胞核抗原(PCNA)表达增强。 西地那非增强血清素诱导的ERK1/ERK2磷酸化,该效应可被MEK抑制剂U0126(10 μmol/L)预处理阻断。 西地那非在1 μmol/L浓度下未能上调MKP-1表达,这可能与其在此条件下缺乏抗增殖作用有关。[2] |
| 体内研究 (In Vivo) |
西地那非显着提高了犬勃起模型中的 ICP 和 ICP/BP,但与媒介物相比,它对血压没有明显影响 [1]。在 10 mg/kg 的剂量下,西地那非治疗显着减少了 TL+- 细胞的数量。 pMCAo 后八天,西地那非治疗(0.5 和/或 10 mg/kg 剂量)显着减少了 Iba-1 染色的小胶质细胞/巨噬细胞的数量 [3]。根据临床前动物模型,西地那非可以通过分泌更多的生长因子(FGF和VEGF)来减少皮瓣坏死。组织学也已证明它对大鼠海绵状神经结构有益[4]。
在出生后第9天(P9)新生小鼠永久性大脑中动脉阻塞(pMCAo)模型中,于缺血后5分钟单次腹腔注射西地那非(10 mg/kg),与PBS相比,并未显著改变早期(至90分钟)的脑血流速度或皮质灌注。 西地那非治疗剂量依赖性地减少了pMCAo后8天测得的平均皮质组织损失:0.5 mg/kg组为16.4 ± 4.8%,10 mg/kg组为11.0 ± 4.8%,而PBS处理组为23.8 ± 7.5%。 在pMCAo后72小时,西地那非(10 mg/kg)显著降低了小胶质细胞/巨噬细胞(番茄凝集素阳性细胞)的总密度并改变了其分布。它增加了半暗带区COX-2+(M1样标志物)细胞的数量,但在核心区没有增加。 在pMCAo后8天,西地那非显著减少了Iba-1+小胶质细胞/巨噬细胞的总数。在10 mg/kg剂量下,它增加了Iba-1+Arg-1+双阳性M2样细胞的比例;在0.5和10 mg/kg剂量下,均减少了Iba-1+COX-2+双阳性M1样细胞的数量。 缺血后8天的基因表达分析显示,与PBS处理相比,西地那非(10 mg/kg)上调了同侧皮质中M1样标志物(CD32, CD86)和多个M2样标志物(CD206, Arg-1, Lgals3, IL1-Rn)的表达。[3] |
| 酶活实验 |
所有关于ERK1/ERK2激活、MKP-1、PCNA表达以及细胞增殖和细胞周期分析的实验都是在第3-5代培养三天的细胞上进行的。此后,细胞在含有0.2%FBS和1%抗生素的RPMI-1640中血清饥饿三天。然后,细胞暴露于1μmol/L的血清素或西地那非,然后暴露于血清素,如图所示。在一些实验中,如所示,细胞在西地那非之前用10μmol/L的U0126预处理30分钟,随后暴露于血清素。在对照组中,用等体积的磷酸盐缓冲盐水(PBS)代替试剂。[2]
ERK1/ERK2磷酸化状态的免疫印迹分析[2] 如上所述,用血清素或1μmol/L的西地那非处理亚融合血清饥饿细胞,然后用或不用U0126刺激血清素。如上所述,在指定时间提取蛋白质。通过蛋白质印迹检测ERK1/ERK2蛋白的磷酸化。简而言之,通过SDS-PAGE分离等量的蛋白质(15-20μg),转移到聚偏二氟乙烯膜上,用抗磷酸化ERK1/ERK2抗体探测,并用辣根过氧化物酶(HRP)偶联的二抗检测。为了测定总ERK1/ERK2的表达,在50°C下用剥离缓冲液洗涤膜30分钟,然后用PBST中的5%牛血清白蛋白封闭膜4小时。此后,用特异性ERK1/ERK2抗体重新探测膜。 MKP-1、PCNA的免疫印迹分析[2] 如上所述,亚融合血清饥饿的PASMC在不同时间段内暴露于西地那非、血清素或U0126。在培养期结束时,提取蛋白质并用12%凝胶进行SDS-PAGE分离。然后将总蛋白转移到聚偏二氟乙烯膜上,在4°C下用PCNA和MKP-1抗体(1:1000)、甘油醛磷酸脱氢酶(GAPDH)抗体(1:2000)检测过夜。洗涤后,在室温下加入适当的二抗(1:5000)一小时。这些印迹是用Super Signal增强化学发光试剂盒开发的,并在柯达AR胶片上可视化。使用图像分析软件通过密度测定对条带进行定量。将蛋白质的相对表达标准化为GAPDH。 |
| 细胞实验 |
MTT比色法[2]
用0.1%胰蛋白酶/0.01%乙二胺四乙酸(EDTA)溶液收获约90%融合的细胞,以2x104个细胞/孔的密度接种到96孔板中,在含有10%FBS的RPMI-1640中生长三天,然后血清饥饿三天。然后将细胞与不同浓度的血清素或1μmol/L的 sildenafil/西地那非孵育不同时间,然后如所示,加入或不加入U0126的血清素。对照细胞以相同的方式处理,除了用无菌PBS代替药物。处理后,将培养基换成新鲜培养基,用5 g/L MTT孵育细胞4小时。然后用150μl 10%二甲亚砜(DMSO)溶解MTT 20分钟。使用微孔板读数器在570nm下测定96孔板中的光密度(OD)。 流式细胞术分析[2] 用0.1%胰蛋白酶/0.01%EDTA收获约90%融合的细胞,以5x104个细胞/孔的密度接种到6孔板中,在含有10%FBS的RPMI-1640中生长3天,然后血清饥饿3天。然后,如所示,将细胞与血清素或1μmol/L的 sildenafil/西地那非一起孵育24小时,然后用或不用U0126刺激血清素。用PBS冲洗细胞,用0.1%胰蛋白酶/0.01%EDTA溶液胰蛋白酶消化,并在20°C下以1000 r/min的速度离心5分钟收集细胞。将细胞颗粒在4°C的70%乙醇中固定至少24小时。用PBS洗涤固定的细胞两次,重新悬浮在含有50g/L RNase A和50mg/L碘化丙啶(PI)的PBS中。将悬浮液在37°C下孵育30分钟,通过200μm尼龙网过滤,然后通过流式细胞仪 进行分析。使用ModfitLT软件进行数据分析。S期细胞与所有G0G1+S+G2M期细胞的比率通过以下公式计算:S期分数(SPF)=S/(G0G1+S/G2M)x100% 采用外植体培养法分离猪PASMCs,使用第3–5代细胞。细胞在含0.2% FBS的RPMI-1640中血清饥饿3天后进行处理。 增殖实验:将细胞接种于96孔板,用血清素(10 μmol/L)处理,部分孔预先用西地那非(1 μmol/L)处理30分钟。3天后进行MTT实验:加入MTT孵育4小时,用DMSO溶解,于570 nm测定吸光度。 细胞周期分析:细胞处理24小时后,用乙醇固定,碘化丙啶和RNase A染色,流式细胞术分析S期细胞比例。 蛋白表达检测:处理后的细胞裂解,Western blot检测磷酸化ERK1/ERK2、总ERK1/ERK2、PCNA和MKP-1表达。[2] |
| 动物实验 |
20 mg/kgSprague-Dawley rats
In the first set of experiments, animals were randomly divided into five groups and treated with either PBS or a single dose of sildenafil citrate (0.5, 2.5, 10, and 15 mg/kg), given intraperitoneally (i.p.) 5 min after pMCAo. In the second set of experiments, animals were randomly divided into three groups and treated with either PBS or a single dose of sildenafil citrate (0.5 and 10 mg/kg, i.p.) 5 min after pMCAo (see Additional file 1: Figure S1 for an outline of the experimental procedure).[3]
cGMP measurement[3] Competitive enzyme immunoassay was used to quantify cGMP in the forebrain, according to the manufacturer’s instructions. Whole brains at P9 were harvested 1 and 3 h after the administration of sildenafil (0.5 and/or 10 mg/kg) and immediately frozen at −80 °C until measurements were performed. Ultrasonographic brain imaging[3] Thermoregulated mice (n = 6 per group) were subjected to ultrasound measurements under inhaled isoflurane anesthesia (0.8 % in air via a facemask) using an echograph equipped with a 14.5-MHz linear transducer (14L5 SP) [12]. Heart rate and time-average mean blood flow velocities (mBFVs) were measured in both intracranial carotid arteries (ICA) and the basilar trunk (BT) at baseline and 1 h after pMCAo and PBS and sildenafil (10 mg/kg) treatment. The study included a total of thirty adult Sprague-Dawley rats that were divided into three groups of ten rats each. In all rats, a crush injury was created by clamping the right sciatic nerve for one minute. One day before the procedure, rats in group 1 were started on a 28-day treatment consisting of a daily dose of 20 mg/kg body weight sildenafil citrate given orally via a nasogastric tube, while the rats in group 2 were started on an every-other-day dose of 10 mg/kg body weight sildenafil citrate. Rats from group 3 were not administered any drugs. Forty-two days after the nerve damage was created, functional and histopathological examination of both sciatic nerves and bone densitometric evaluation of the extremities were conducted.[4] Permanent middle cerebral artery occlusion (pMCAo) was performed on postnatal day 9 (P9) C57Bl/6 mouse pups (4.6 ± 0.6 g) under isoflurane anesthesia. Sildenafil citrate was dissolved in PBS. A single intraperitoneal injection was administered 5 minutes after pMCAo. Animals were randomly assigned to treatment groups receiving PBS or sildenafil at doses of 0.5, 2.5, 10, or 15 mg/kg. For hemodynamic assessment, cerebral blood flow was measured at baseline and at various time points up to 90 minutes post-pMCAo using color-coded pulsed Doppler ultrasound imaging of large cerebral arteries and laser speckle contrast imaging of cortical perfusion. Animals were euthanized at 72 hours or 8 days after pMCAo for brain tissue collection. Lesion volume was determined on brain sections. Brain tissues were processed for RNA extraction (qRT-PCR), protein analysis (immunohistochemistry, immunofluorescence), and cGMP measurement (competitive enzyme immunoassay).[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Sildenafil is known to be quickly absorbed, with maximum plasma concentrations being observed within 30-120 minutes (with a median of 60 minutes) of oral administration in a fasting patient. Moreover, the mean absolute bioavailability observed for sildenafil is about 41% (from a range of 25-63%). In particular, after oral three times a day dosing of sildenafil, the AUC and Cmax increase in proportion with dose over the recommended dosage range of 25-100 mg. When used in pulmonary arterial hypertension patients, however, the oral bioavailability of sildenafil after a dosing regimen of 80 mg three times a day, was on average 43% greater than compared to the lower doses. Finally, if sildenafil is administered orally with food, the rate of absorption is observed to be decreased with a mean delay in Tmax of about 60 minutes and a mean decrease in Cmax of approximately 29%. Regardless, the extent of absorption is not observed to be significantly affected as the recorded AUC decreased by only about 11 %. After either oral or intravenous administration, sildenafil is excreted as metabolites predominantly in the feces (approximately 80% of the administered oral dose) and to a lesser extent in the urine (approximately 13% of the administered oral dose). The mean steady-state volume of distribution documented for sildenafil is approximately 105 L - a value which suggests the medication undergoes distribution into the tissues. The total body clearance documented for sildenafil is 41 L/h. Sildenafil is rapidly and almost completely absorbed following oral administration. Bioequivalence has been established between the 20-mg tablet and the 10-mg/mL oral suspension when administered as a single oral dose of 20 mg. Although single-dose studies indicate that more than 90% of an oral sildenafil dose is absorbed from the GI tract, the drug undergoes extensive metabolism in the GI mucosa during absorption and on first pass through the liver, with only about 40% of a dose reaching systemic circulation unchanged. Pharmacokinetics of the drug (as determined by peak plasma concentrations or area under the plasma concentration-time curve (AUC)) are dose proportional over the single-dose range of 1.25-200 mg. Peak plasma concentrations of sildenafil and its active N-desmethyl metabolite are achieved within 30-120 (median: 60) minutes following oral administration in fasting adults. Sildenafil appears to be widely distributed in the body, with a reported volume of distribution at steady state averaging 105 L. It is not known whether sildenafil is distributed into milk. Sildenafil and its major circulating N-desmethyl metabolite are each approximately 96% bound to plasma proteins; protein binding reportedly is independent of plasma concentration over the range of 0.01-10 ug/mL. Plasma protein binding of the drug in geriatric adults older than 65 years of age is slightly greater (97%) than that observed in individuals younger than 45 years of age (96%). Sildenafil is distributed to a limited extent in semen following oral administration, with less than 0.001% of a single dose appearing in semen 90 minutes after dosing in healthy individuals Such concentrations are unlikely to cause any effects in sexual partners exposed to the semen. Sildenafil is eliminated mainly in the feces as metabolites. In healthy adults and those with erectile dysfunction, approximately 80% of an oral dose is excreted as metabolites in feces and 13% is excreted in urine. In volunteers with mild (CLcr=50-80 mL/min) and moderate (CLcr=30-49 mL/min) renal impairment, the pharmacokinetics of a single oral dose of Viagra (50 mg) were not altered. In volunteers with severe (CLcr=<30 mL/min) renal impairment, sildenafil clearance was reduced, resulting in approximately doubling of AUC and Cmax compared to age-matched volunteers with no renal impairment. For more Absorption, Distribution and Excretion (Complete) data for SILDENAFIL (10 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of sildenafil is facilitated primarily by the CYP3A4 hepatic microsomal isoenzymes and to a minor extent, via the CYP2C9 hepatic isoenzymes. The predominant circulating metabolite results from the N-demethylation of sildenafil. This particular resultant metabolite possesses a phosphodiesterase selectivity that is similar to the parent sildenafil molecule and a corresponding in vitro potency for PDE5 that is approximately 50% that of the parent drug. Moreover, plasma concentrations of the metabolite are about 40% of those recorded for sildenafil, a percentage that accounts for about 20% of sildenafil’s pharmacologic effects. This primary N-desmethyl metabolite of sildenafil also undergoes further metabolism, with a terminal half-life of about 4 hours. In patients with pulmonary arterial hypertension, plasma concentrations of the primary N-desmethyl metabolite are about 72% those of the original parent sildenafil molecule after a regimen of 20 mg three times a day - which is consequently responsible for about a 36% contribution to sildenafil’s overall pharmacological effects. Sildenafil is cleared predominantly by the CYP3A4 (major route) and CYP2C9 (minor route) hepatic microsomal isoenzymes. The major circulating metabolite results from N-desmethylation of sildenafil, and is itself further metabolized. This metabolite has a phosphodiesterase (PDE) selectivity profile similar to sildenafil and an in vitro potency for phosphodiesterase type 5 (PDE-5) approximately 50% of the parent drug. Plasma concentrations of this metabolite are approximately 40% of those seen for sildenafil, so that the metabolite accounts for about 20% of sildenafil's pharmacologic effects. Pharmacokinetics were studied in mouse, rat, rabbit, dog and man after single intravenous and/or oral doses of sildenafil or (14)C-sildenafil (Viagra). .... Five principal pathways of metabolism in all species were piperazine N-demethylation, pyrazole N-demethylation, loss of a two-carbon fragment from the piperazine ring (N,N'-deethylation), oxidation of the piperazine ring and aliphatic hydroxylation. Additional metabolites arose through combinations of these pathways. Sildenafil was the major component detected in human plasma. Following oral doses, AUC (infinity) for the piperazine N-desmethyl and piperazine N,N'-desethyl metabolites were 55 and 27% that of parent compound respectively. Sildenafil is eliminated mainly in the feces as metabolites. In healthy adults and those with erectile dysfunction, approximately 80% of an oral dose is excreted as metabolites in feces and 13% is excreted in urine. In feces, the N-dealkylated, hydroxylated, N-demethylated, and N-dealkylated/demethylated metabolites of sildenafil comprise about 22, 13, 3, and 3% of total fecal excretion. In healthy individuals, sildenafil is excreted in urine mainly as the hydroxylated metabolite, with this metabolite representing about 41% of total urinary excretion of the drug. /Sprague Dawley rats (10/sex/dose) were administered 10, 45 or 200 mg/kg/day of sildenafil for 1 month by oral gavage./ Plasma concentrations of sildenafil were higher in females than in males, while concentrations of the metabolite, UK-103,320, were higher in males than in females. As a result, females were exposed predominantly to the unchanged drug and males to an almost equal balance of drug and metabolite. These data indicate that N-demethylation of sildenafil to UK-103,320 is an important route of sildenafil biotransformation in male rats. Concentrations of UK-95,340 were generally below the limit of determination (30 ng/mL). ... /From table/ Sildenafil appears to be completely metabolized in the liver to up to 16 metabolites, most of which represent only a small fraction of a dose; little or no unchanged drug is detectable in urine or feces following oral or IV administration. Sildenafil is metabolized principally via hepatic cytochrome P-450 (CYP) microsomal isoenzymes 3A4 (major route) and 2C9 (minor route), and potent inhibitors of CYP3A4 can substantially reduce sildenafil clearance. Hepatic metabolism of sildenafil is complex, generally involving the piperazine ring, N,N-de-ethylation (ring opening) or N-demethylation of the piperazine ring and aliphatic hydroxylation; the drug and its metabolites do not appear to undergo conjugation. The N-demethylated metabolite, the major circulating metabolite, has a phosphodiesterase selectivity profile similar to that of sildenafil and an in vitro potency for PDE type 5 of approximately 50% of the parent drug. The N-demethylated metabolite is further metabolized to an N-dealkylated N,N-de-ethylated) metabolite. The drug also undergoes N-dealkylation followed by N-demethylation of the piperazine ring. Biological Half-Life The terminal phase half-life observed for sildenafil is approximately 3 to 5 hours. Plasma sildenafil concentrations appear to decline in a biphasic manner following oral administration, with a terminal elimination half-life of about 4 hours (range: 3-5 hours). High clearance was the principal determinant of short elimination half-lives in rodents (0.4-1.3 hr), whereas moderate clearance in dog and man resulted in longer half-lives (6.1 and 3.7 hr respectively). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sildenafil is a white to off-white crystalline powder that is formulated into film-coated tablets, oral suspension, and parenteral injection. Sildenafil is a phosphodiesterase-5 (PDE-5) inhibitor. It is used both for the treatment of erectile dysfunction and for the treatment of pulmonary arterial hypertension (PAH) in adults to improve exercise ability and delay clinical worsening. HUMAN EXPOSURE AND TOXICITY: In general, overdosage of sildenafil may be expected to produce effects that are extensions of common adverse reactions. In studies of healthy individuals receiving single sildenafil doses up to 800 mg, the types of adverse events (e.g., decreased blood pressure, syncope, and prolonged erection) observed were similar to those observed at lower doses, but the incidences were increased. Serious adverse effects have also been reported at therapeutic dose levels including sudden decrease or loss of hearing, sudden loss of vision in one or both eyes, and prolonged erection lasting greater than 4 hours or priapism (a painful erection lasting greater than 6 hours). Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of sildenafil for erectile dysfunction. Most, but not all, of these patients had preexisting cardiovascular risk factors. Therefore it was not possible to determine whether these events were related directly to sildenafil, to sexual activity, to the patient's underlying cardiovascular disease, to a combination of these factors, or to other factors. The use of sildenafil is not recommended in children. In a long-term trial in pediatric patients with PAH, an increase in mortality with increasing sildenafil dose was observed. Pulmonary vasodilators such as sildenafil may significantly worsen the cardiovascular status of patients with pulmonary veno-occlusive disease. Sildenafil profoundly potentiates the vasodilatory effects of organic nitrates and nitrites. The drug did not exhibit clastogenic potential in an in vitro human lymphocytes test system. ANIMAL STUDIES: Lethality after oral administration occurred at 1000 mg/kg and 500 mg/kg in rats and 1000 mg/kg in mice. Female rats were more affected than male rats. Acute sildenafil treatment stimulated testosterone production in adult male rats. There was no impairment of fertility in rats given sildenafil up to 60 mg/kg/day for 36 days to females and 102 days to males. However, in another study male rats were gavaged with sildenafil citrate (0.06 mg/0.05 mL) and allowed to mate. Fertilization rates and numbers of embryos were evaluated after treatment. Fertilization rates (day 1) were markedly reduced (approximately 33%) in matings where the male had taken sildenafil citrate. Over days 2-4, the numbers of embryos developing in the treated group were significantly fewer than in the control group. There was also a trend for impaired cleavage rates within those embryos, although this did not reach significance. No evidence of teratogenicity, embryotoxicity or fetotoxicity was observed in rats and rabbits which received up to 200 mg/kg/day during organogenesis. In another study, adult male rabbits received sildenafil at doses up to 9 mg/kg/day for 4 weeks to investigate the testicular histological alterations induced by overdoses of this drug. Abnormality in the germinal epithelium of the seminiferous tubules included spermatocytes karyopyknosis, spermatocytes degeneration, desquamation, spermatid giant cells and arrest of spermatogenesis. Additionally, increased Leydig cells cellularity, tubular degeneration, thickening of the interstitium were also observed. The encountered histological findings indicate that chronic exposure to sildenafil overdoses produces significant morphological and histological alterations in the testes which finally might lead to complete arrest of spermatogenesis. There was no evidence of carcinogenicity when sildenafil was administered orally to rats and mice for up to two years. Sildenafil did not exhibit evidence of mutagenicity in vitro in bacterial and Chinese hamster ovary cell assays. The drug also did not exhibit clastogenic potential in vivo in the mouse micronucleus test. Sildenafil is cleared predominantly by the CYP3A4 (major route) and CYP2C9 (minor route) hepatic microsomal isoenzymes. The major circulating metabolite results from N-desmethylation of sildenafil, and is itself further metabolized. This metabolite has a phosphodiesterase (PDE) selectivity profile similar to sildenafil and an in vitro potency for phosphodiesterase type 5 (PDE-5) approximately 50% of the parent drug. Plasma concentrations of this metabolite are approximately 40% of those seen for sildenafil, so that the metabolite accounts for about 20% of sildenafil's pharmacologic effects. Hepatotoxicity There have been at least 5 reports of acute liver injury attibuted to sildenafil use, but no instances of acute hepatic failure. The latency in most reports has been unclear because of the intermittent and sometimes unacknowledged use of sildenafil, but appears to be within 1 to 8 weeks. The pattern of serum enzyme elevations has ranged from hepatocellular to cholestatic, sometimes evolving from one to the other. The most convincing cases have been a mild cholestatic or \"mixed\" hepatitis arising within 1 to 3 months of starting sildenafil. Immunoallergic features and autoantibodies were not observed. Cases of acute onset with high serum aminotransferase levels have been reported after use of sildenafil that have some characteristics of ischemic injury. In other instances, the pattern of injury suggested anabolic steroid use. In two cases, re-exposure did not result in recurrence. Thus, the hepatotoxicity of sildenafil is not completely convincing and must be quite rare, if it occurs at all. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited data indicate that sildenafil and its active metabolite in breastmilk are poorly excreted into breastmilk. Amounts ingested by the infant are far below doses given to treat infants and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants A 23-year-old woman with congenital heart disease and pulmonary hypertension was treated during pregnancy with sildenafil and bosentan in unspecified dosages. These drugs and warfarin were continued postpartum. Her infant was delivered at 30 weeks by cesarean section and weighed 1.41 kg at birth. She nursed the infant in the neonatal intensive care unit for 11 weeks \"with good outcome\" according to the authors, but the infant died at 26 weeks from a respiratory syncytial virus infection.[3] A woman breastfeeding her 21-month-old infant was taking 20 mg of sildenafil 3 times daily and 125 mg of bosentan twice daily to treat pulmonary arterial hypertension. The drugs were begun more than 6 months postpartum. The mother did not report any possible adverse effects, serious health problems or hospitalization of the infant in the period from birth until day 651 postpartum when the infant was partially breastfed.[2] ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding It is generally observed that sildenafil and its main circulating N-desmethyl metabolite are both estimated to be about 96% bound to plasma proteins. Nevertheless, it has been determined that protein binding for sildenafil is independent of total drug concentrations. Interactions Sildenafil and other phosphodiesterase (PDE) type 5 inhibitors (e.g., tadalafil, vardenafil) profoundly potentiate the vasodilatory effects (e.g., a systolic blood pressure reduction exceeding 25 mm Hg with sildenafil) of organic nitrates and nitrites (e.g., nitroglycerin, isosorbide dinitrate), and potentially life-threatening hypotension and/or hemodynamic compromise can result. Nitrates and nitrites promote the formation of cyclic guanosine monophosphate (cGMP) by stimulating guanylate cyclase, and PDE type 5 inhibitors (e.g., sildenafil, tadalafil, vardenafil) act to decrease the degradation of cGMP via phosphodiesterase (PDE) type 5 by inhibiting this enzyme, resulting in increased accumulation of cGMP and more pronounced smooth muscle relaxation and vasodilation than with either PDE type 5 inhibitors or nitrates/nitrites alone. This interaction probably occurs with any organic nitrate, nitrite, or nitric oxide donor (e.g., nitroprusside) regardless of their predominant hemodynamic site of action. PDE type 5 inhibitors /including sildenafil/ also may potentiate the hypotensive effects of inhaled nitrites (e.g., amyl or butyl nitrite, sometimes referred to as poppers), which may be misused (recreational use) during sexual activity for purported effects in enhancing the sexual experience. Because these agents are used recreationally, patients may be unaware of their pharmacologic effects and potential risks and may not report their use to clinicians. Concurrent use of PDE type 5 inhibitors with poppers, which dilate blood vessels with a rapid onset of action, could result in sudden and marked blood pressure reduction and potentially serious or even fatal effects. Interactions with organic nitrates and nitrites may be even more pronounced in patients who also are taking certain HIV protease inhibitors concomitantly. Homosexual males may be at particular risk because of the greater likelihood of recreational inhaled nitrite use and antiretroviral therapy in this population. Combination antiretroviral therapy usually includes one or more HIV protease inhibitors that are inhibitors of CYP3A4 and/or CYP2C9, and the possibility for an interaction with sildenafil clearance resulting in an increased likelihood of sildenafil-associated adverse effects such as headache, flushing, visual changes, priapism, and possibly hypotension and syncope exists. Patients should inform their clinician if they are taking antiretroviral therapy. Sildenafil potentiates the hypotensive effect of nitrates /including nitroglycerin/; concomitant use is contraindicated. For more Interactions (Complete) data for SILDENAFIL (23 total), please visit the HSDB record page. A high dose of sildenafil (15 mg/kg, single i.p. injection) administered 5 minutes after pMCAo in P9 mice significantly increased mean cortical infarct volume at 72 hours (21.6 ± 4.6%) compared to PBS (12.5 ± 3.0%) and increased mortality (3 out of 10 animals died), suggesting toxicity at this dose.[3] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Phosphodiesterase 5 Inhibitors; Urological Agents; Vasodilator Agents Viagra is indicated for the treatment of erectile dysfunction. /Included in US product labeling/ Revatio is indicated for the treatment of pulmonary arterial hypertension in adults to improve exercise ability and delay clinical worsening. /Included in US product label/ The role, if any, of sildenafil in the management of sexual dysfunction in women remains to be established. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for SILDENAFIL (9 total), please visit the HSDB record page. Drug Warnings Administration of Viagra with nitric oxide donors such as organic nitrates or organic nitrites in any form is contraindicated. Consistent with its known effects on the nitric oxide/cGMP pathway, Viagra was shown to potentiate the hypotensive effects of nitrates. Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of Viagra. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of Viagra without sexual activity. Others were reported to have occurred hours to days after the use of Viagra and sexual activity. It is not possible to determine whether these events are related directly to Viagra, to sexual activity, to the patient's underlying cardiovascular disease, to a combination of these factors, or to other factors. Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of Viagra. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency could result. Angina pectoris, AV block, tachycardia, palpitation, myocardial ischemia and infarction, sudden cardiac death, chest pain, cerebral thrombosis, cerebrovascular hemorrhage (e.g., subarachnoid, intracerebral hemorrhage), transient ischemic attack, stroke (e.g., hemorrhagic or brainstem), cardiac or cardiopulmonary arrest, coronary artery disease, heart failure, electrocardiographic (ECG) abnormalities including ventricular arrhythmia (e.g., tachycardia, premature complexes) or Q-wave abnormalities (without myocardial infarction), hypertension, edema (including facial and peripheral), shock, and cardiomyopathy also have occurred in less than 2% of patients with erectile dysfunction receiving sildenafil in controlled clinical trials and in postmarketing surveillance, but have not been directly attributed to the drug. The incidence of myocardial infarction or stroke was similar in patients receiving sildenafil for the treatment of erectile dysfunction or placebo, and most cases occurred within a few hours to days after a sildenafil dose or placebo. Most patients experiencing serious adverse cardiovascular effects had preexisting cardiovascular risk factors, and many of these effects were reported to occur shortly after taking sildenafil, either with or without sexual activity. In at least one patient with hypertrophic cardiomyopathy, decreased blood pressure, marked reductions in ventricular dimensions, increased ejection fraction and subaortic gradient at rest, ventricular premature complexes, and unsustained ventricular tachycardia occurred following sildenafil administration for the treatment of erectile dysfunction. For more Drug Warnings (Complete) data for SILDENAFIL (32 total), please visit the HSDB record page. Pharmacodynamics In vitro studies have shown that sildenafil is selective for phosphodiesterase-5 (PDE5). Its effect is more potent on PDE5 than on other known phosphodiesterases. In particular, there is a 10-times selectivity over PDE6 which is involved in the phototransduction pathway in the retina. There is an 80-times selectivity over PDE1, and over 700-times over PDE 2, 3, 4, 7, 8, 9, 10 and 11. And finally, sildenafil has greater than 4,000-times selectivity for PDE5 over PDE3, the cAMP-specific phosphodiesterase isoform involved in the control of cardiac contractility. In eight double-blind, placebo-controlled crossover studies of patients with either organic or psychogenic erectile dysfunction, sexual stimulation resulted in improved erections, as assessed by an objective measurement of hardness and duration of erections (via the use of RigiScan®), after sildenafil administration compared with placebo. Most studies assessed the efficacy of sildenafil approximately 60 minutes post-dose. The erectile response, as assessed by RigiScan®, generally increased with increasing sildenafil dose and plasma concentration. The time course of effect was examined in one study, showing an effect for up to 4 hours but the response was diminished compared to 2 hours. Sildenafil causes mild and transient decreases in systemic blood pressure which, in the majority of cases, do not translate into clinical effects. After chronic dosing of 80 mg, three times a day to patients with systemic hypertension the mean change from baseline in systolic and diastolic blood pressure was a decrease of 9.4 mmHg and 9.1 mmHg respectively. After chronic dosing of 80 mg, three times a day to patients with pulmonary arterial hypertension lesser effects in blood pressure reduction were observed (a reduction in both systolic and diastolic pressure of 2 mmHg) . At the recommended dose of 20 mg three times a day no reductions in systolic or diastolic pressure were seen. Single oral doses of sildenafil up to 100 mg in healthy volunteers produced no clinically relevant effects on ECG. After chronic dosing of 80 mg three times a day to patients with pulmonary arterial hypertension no clinically relevant effects on the ECG were reported either. In a study of the hemodynamic effects of a single oral 100 mg dose of sildenafil in 14 patients with severe coronary artery disease (CAD) (> 70 % stenosis of at least one coronary artery), the mean resting systolic and diastolic blood pressures decreased by 7 % and 6 % respectively compared to baseline. Mean pulmonary systolic blood pressure decreased by 9%. Sildenafil showed no effect on cardiac output and did not impair blood flow through the stenosed coronary arteries. Mild and transient differences in color discrimination (blue/green) were detected in some subjects using the Farnsworth-Munsell 100 hue test at 1 hour following a 100 mg dose, with no effects evident after 2 hours post-dose. The postulated mechanism for this change in color discrimination is related to inhibition of PDE6, which is involved in the phototransduction cascade of the retina. Sildenafil has no effect on visual acuity or contrast sensitivity. In a small size placebo-controlled study of patients with documented early age-related macular degeneration (n = 9), sildenafil (single dose, 100 mg) demonstrated no significant changes in visual tests conducted (which included visual acuity, Amsler grid, color discrimination simulated traffic light, and the Humphrey perimeter and photostress test). Sildenafil is a selective PDE5 inhibitor that increases intracellular cGMP levels. In this neonatal stroke model, the neuroprotective effect of sildenafil in reducing late-phase lesion extension appears to be independent of early hemodynamic changes but involves modulation of the post-ischemic inflammatory response. The study suggests that sildenafil promotes a shift in microglia/macrophage polarization towards a neuroprotective M2-like phenotype in the late phase after ischemia. Sildenafil is indicated for idiopathic pulmonary hypertension and could be explored as a potential immunomodulatory treatment for neonatal ischemic stroke.[3] |
| 分子式 |
C22H30N6O4S
|
|---|---|
| 分子量 |
474.5764
|
| 精确质量 |
474.204
|
| 元素分析 |
C, 55.68; H, 6.37; N, 17.71; O, 13.48; S, 6.76
|
| CAS号 |
139755-83-2
|
| 相关CAS号 |
Sildenafil citrate;171599-83-0;Sildenafil-d3;1126745-90-1;Sildenafil-d8;951385-68-5;Sildenafil Mesylate;1308285-21-3;Sildenafil-d3N-1;1126745-87-6
|
| PubChem CID |
135398744
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
189-190 °C
187-189 °C |
| LogP |
1.5
|
| tPSA |
118
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
838
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1C([H])=C([H])C(=C(C2=NC3C(C([H])([H])C([H])([H])C([H])([H])[H])=NN(C([H])([H])[H])C=3C(N2[H])=O)C=1[H])OC([H])([H])C([H])([H])[H])(N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])(=O)=O
|
| InChi Key |
BNRNXUUZRGQAQC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)
|
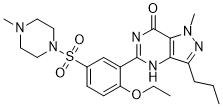
| 化学名 |
Piperazine, 1-((3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo(4,3-d)pyrimidin-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methyl-
|
| 别名 |
UK-92480; UK 92480-10; UK 92480; UK92480 citrate; UK-92,480-10. Trade names: Revatio.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.27 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol:30 mg/mL 配方 4 中的溶解度: ≥ 10 mg/mL (21.07 mM) (饱和度未知) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1071 mL | 10.5356 mL | 21.0713 mL | |
| 5 mM | 0.4214 mL | 2.1071 mL | 4.2143 mL | |
| 10 mM | 0.2107 mL | 1.0536 mL | 2.1071 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05558176 | Recruiting | Drug: Sildenafil citrate | Foetal Hypoxia | Ladoke Akintola University of Technology Teaching Hospital, Ogbomoso |
April 8, 2022 | Phase 4 |
| NCT02845388 | Completed | Drug: Sildenafil citrate Drug: estradiol valerate |
Infertility | Omar Ahmed El Sayed Saad | September 2015 | Phase 2 |
| NCT05951413 | Recruiting | Drug: Sildenafil Citrate Drug: estradiol |
IVF | Beni-Suef University | June 30, 2023 | Phase 2 Phase 3 |
| NCT03417492 | Terminated | Drug: Sildenafil Citrate | Traumatic Brain Injury Mild Traumatic Brain Injury |
University of Pennsylvania | March 1, 2018 | Phase 1 |
Sildenafil enhances the lethality of [pemetrexed + sorafenib].Oncotarget. 2017 Feb 21; 8(8): 13464–13475. |
[Pemetrexed + sorafenib + sildenafil] treatment inactivates cyto-protective STAT3, STAT5 and AKT whilst reducing the expression of cyto-protective proteins MCL-1, BCL-XL and Thioredoxin.Oncotarget. 2017 Feb 21; 8(8): 13464–13475. |
Sildenafil-induced PKG signaling plays a greater role in enhancing [pemetrexed + sorafenib] toxicity than nitric oxide synthase signaling.Oncotarget. 2017 Feb 21; 8(8): 13464–13475. |