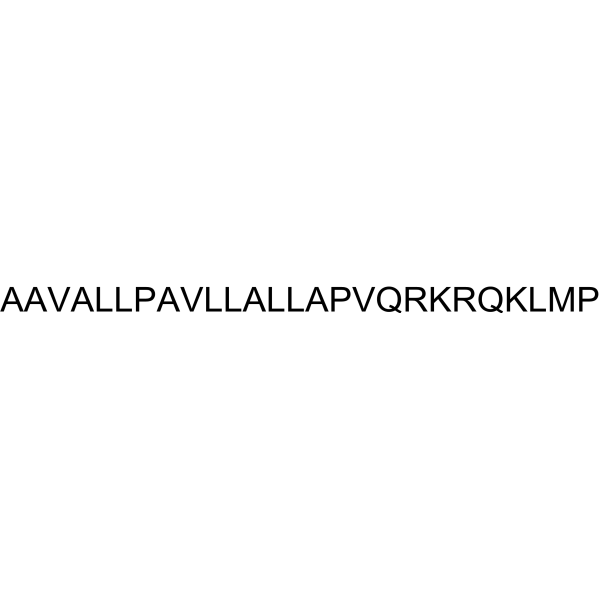
| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
NF-κB
|
|---|---|
| 体外研究 (In Vitro) |
与使用媒介物治疗的组相比,SN50 预处理会导致 TBI 后 12、24 和 48 小时的 PI 阳性细胞数量显着减少[1]。局部使用 SN50 通过抑制局部细胞中核因子-κB 的激活,降低角膜愈合过程中上皮缺陷和溃疡的发生率。与对照组相比,治疗后的角膜中肌成纤维细胞的产生、巨噬细胞浸润、基质金属蛋白酶活性、基底膜破裂和细胞因子表达均有所减少[2]。用SN50处理人胃癌细胞SGC7901可能会显着增强LY294002在24小时后诱导细胞死亡的作用[3]。 LPS 诱导的肺损伤与 NF-kB 的易位和炎症细胞因子的产生有关,SN50 可以抑制这两者[4]。
本研究发现,与对照组相比,用SN50治疗人癌症细胞SGC7901可显著增强LY294002在24小时后诱导细胞死亡的作用(p<0.05)。线粒体电位检测和透射电镜检查表明,细胞死亡率逐渐增加。p53、PUMA和Beclin1的表达上调。 结论:NF-κB抑制剂SN50可增强LY294002诱导人癌症细胞SGC7901细胞死亡的作用,这可能是一种很有前途的治疗癌症的新途径。[3] |
| 体内研究 (In Vivo) |
从第一天到第四天,SN50治疗可加速运动功能结果的恢复。 TBI 后 7 天和 8 天,接受 SN50 预处理的动物与对照组相比,其视觉空间学习潜伏期显着降低。 SN50预处理后,TBI后12~48小时和TBI后6~48小时,TNF-a和NF-κB p65蛋白水平分别显着降低[1]。
|
| 酶活实验 |
线粒体电位(ΔΨ)的检测[3]
使用KeyGEN线粒体膜传感器试剂盒测定线粒体ΔΨ。Mitosensor染料聚集在健康细胞的线粒体中,在绿色单体细胞质背景染色下发出红色荧光。然而,在线粒体ΔΨ坍塌的细胞中,染料不能在线粒体中积累,而是以单体形式在整个细胞中保持绿色荧光。简而言之,将SGC7901细胞与LY294002、SN50和LY294002+SN50在6孔板中孵育指定时间,然后沉淀,用PBS洗涤,并重新悬浮在0.5ml稀释的Mitosensor试剂(在孵育缓冲液中为1µmol/ml)中。用Mitosensor试剂孵育细胞20分钟后,加入0.2 ml孵育缓冲液,离心细胞,然后重新悬浮在40µl孵育缓冲溶液中。最后,洗涤细胞并重新悬浮在1ml PBS中用于流式细胞术分析。 p53、Beclin1和PUMA的实时定量RT-PCR分析[3] 使用RNAiso试剂盒提取总RNA。为了提取总mRNA,SGC7901细胞在收获前用LY294002(50µmol/l)、SN50(18µmol/l)和LY294002+SN50处理6小时。根据制造商的说明,使用随机引物和Primescript RT试剂盒在总反应体积为20µl的条件下,通过逆转录2µg总RNA生成第一链cDNA。使用Primer5软件(可从frodo.wi.mit.edu/cgi-bin/Primer5/Primer5_www.cgi获得)设计对所选候选基因和管家基因具有特异性的正向和反向寡核苷酸引物的序列。p53的引物为:正向5'-ACTAAGCG-AGCACTGCCCAAC-3';反向,5'-CCTCATTCA-GCTCGGAACATC-3'。对于PUMA,引物为:正向5'-CGACCTCACGACAGTACGA-3';反向,5'-GGCACATTGGGCTCCATC-3'。对于Beclin1,引物为:正向5'-CCAGATGC-GTTATGCCCAGAC-3';反向,5'-CATTCCATTC-CACGGGAACAC-3'。β-actin的引物为:正向5'-ATTGCCGACAGGATGCAGA-3';反向,5'-GAGTACTTGCGCTCAGGAGGA-3'。使用iCycler 5热循环仪进行实时定量RT-PCR。使用SYBR Premix EX Taq试剂盒,在20µl体积内扩增每种cDNA的80倍稀释液,每种引物的终浓度为500 nM。使用熔解曲线分析检查扩增特异性。阈值周期Ct与靶mRNA水平呈负相关,使用Light Cycler软件提供的二阶导数最大值算法计算。对于每个cDNA,所有靶基因mRNA水平均归一化为β-actin mRNA水平。结果表示为处理细胞中归一化靶基因mRNA水平相对于未处理细胞中的比率。 透射电子显微镜检查[3] 根据LY294002、SN50或LY294002+SN50的处理,将细胞固定在0.1 M PBS中的2.5%戊二醛中,并在4°C下保存以供进一步处理。当处理恢复时,将细胞在相同缓冲液中的1%四氧化锇中后固定,在分级醇中脱水,包埋在Epon 812中,用超微切片机切片,用乙酸铀酰和柠檬酸铅染色,然后用透射电子显微镜检查。 |
| 细胞实验 |
细胞活力测定[3]
用MTT法评估细胞存活率。为了确定SN50对增强LY294002对SGC7901细胞作用的影响,将细胞铺入96孔微孔板(7×103个细胞/孔)中并培养24小时。然后将LY294002(50µmol/l)、SN50(18µmol/l。随后在处理结束前4小时将MTT溶液加入培养基(终浓度为5mg/ml)中,并通过加入10%酸化的SDS 100µl停止反应。使用自动多孔分光光度计读取570nm处的吸光度值(A)。细胞死亡百分比计算如下:细胞死亡(%)=(1-A实验孔/A阳性对照孔)×100%。 采用3-(4,5-二甲基噻唑-2-基-2,5-二苯基溴化四唑(MTT)法测定药物的细胞毒性作用。使用荧光探针JC-1测量线粒体膜电位。Hoechst 33258染色用于检测LY294002和/或SN50处理后的凋亡和坏死形态学变化。实时聚合酶链式反应(RT-PCR)分析p53、PUMA和Beclin1的表达。我们使用透射电子显微镜来鉴定LY294002和/或SN50处理后SGC7901细胞的超微结构变化[3]。 |
| 动物实验 |
The present study sought to first investigate the effect of a NF-κB inhibitor SN50, which inhibits NF-κB nuclear translocation, on cell death and behavioral deficits in our mice traumatic brain injury (TBI) models. Additionally, we tried to elucidate the possible mechanisms of the therapeutic effect of SN50 through NF-κB regulating apoptotic and inflammatory pathway in vivo. Encouragingly, the results showed that pretreatment with SN50 remarkably attenuated TBI-induced cell death (detected by PI labeling), cumulative loss of cells (detected by lesion volume), and motor and cognitive dysfunction (detected by motor test and Morris water maze). To analyze the mechanism of SN50 on cell apoptotic and inflammatory signaling pathway, we thus assessed expression levels of TNF-α, cathepsin B and caspase-3, Bid cleavage and cytochrome c release in SN50-pretreated groups compared with those in saline vehicle groups. The results imply that through NF-κB/TNF-α/cathepsin networks SN50 may contribute to TBI-induced extrinsic and intrinsic apoptosis, and inflammatory pathways, which partly determined the fate of injured cells in our TBI model.[1]
Researchers evaluated the therapeutic efficacy of topical administration of SN50, an inhibitor of nuclear factor-kappaB, in a corneal alkali burn model in mice. An alkali burn was produced with 1 N NaOH in the cornea of C57BL/6 mice under general anesthesia. SN50 (10 microg/microl) or vehicle was topically administered daily for up to 12 days. The eyes were processed for histological or immunohistochemical examination after bromodeoxyuridine labeling or for semi-quantification of cytokine mRNA. Topical SN50 suppressed nuclear factor-kappaB activation in local cells and reduced the incidence of epithelial defects/ulceration in healing corneas. Myofibroblast generation, macrophage invasion, activity of matrix metalloproteinases, basement membrane destruction, and expression of cytokines were all decreased in treated corneas compared with controls. To elucidate the role of tumor necrosis factor (TNF)-alpha in epithelial cell proliferation, we performed organ culture of mouse eyes with TNF-alpha, SN50, or an inhibitor of c-Jun N-terminal kinase (JNK) and examined cell proliferation in healing corneal epithelium in TNF-alpha-/- mice treated with SN50. An acceleration of epithelial cell proliferation by SN50 treatment was found to depend on TNF-alpha/JNK signaling. In conclusion, topical application of SN50 is effective in treating corneal alkali burns in mice.[2] |
| 参考文献 |
|
| 其他信息 |
SN50 is a 26-amino acid peptide with formula C129H230N36O29S. It is a cell permeable inhibitor of NF-kappaB translocation. It has a role as a NF-kappaB inhibitor, a cardioprotective agent and an anti-inflammatory agent.
NF-κB cell permeable inhibitory peptide (SN50) inhibits translocation of nuclear factor-κB (NF-κB) and production of inflammatory cytokines that are implicated in lipopolysaccharide (LPS)-induced lung injury (LPSLI). However, the protective effect of SN50 in LPSLI is unclear. We explored the cellular and molecular mechanisms of SN50 treatment in LPSLI. LPSLI was induced by intratracheal instillation of 10 mg/kg LPS using an isolated and perfused rat lung model. SN50 was administered in the perfusate 15 minutes before LPS was administered. Hemodynamics, lung histologic change, inflammatory responses, and activation of apoptotic pathways were evaluated. After LPSLI, increased pulmonary vascular permeability and lung weight gain was observed. The levels of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, myeloperoxidase, and macrophage inflammatory protein-2 increased in bronchoalveolar lavage fluids. Lung-tissue expression of TNF-α, IL-1β, mitogen-activated protein kinases (MAPKs), caspase-3, p-AKT (serine-threonine kinase, also known as protein kinase B), and plasminogen activator inhibitor-1 (PAI-1) was greater in the LPS group compared with controls. Upregulation and activation of NF-κB was associated with increased lung injury in LPSLI. SN50 attenuated the inflammatory responses, including expression of IL-1β, TNF-α, myeloperoxidase, MAPKs, PAI-1, and NF-κB; downregulation of apoptosis indicated by caspase-3 and p-AKT expression was also observed. In addition, SN50 mitigated the increase in the lung weight, pulmonary vascular permeability, and lung injury. In conclusion, LPSLI is associated with inflammatory responses, apoptosis, and coagulation. NF-κB is an important therapeutic target in the treatment of LPSLI. SN50 inhibits translocation of NF-κB and attenuates LPSLI.[4] |
| 分子式 |
C129H230N36O29S
|
|---|---|
| 分子量 |
2781.49532842636
|
| 精确质量 |
2779.74
|
| CAS号 |
213546-53-3
|
| PubChem CID |
16209942
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
9.812
|
| tPSA |
1051.77
|
| 氢键供体(HBD)数目 |
34
|
| 氢键受体(HBA)数目 |
35
|
| 可旋转键数目(RBC) |
92
|
| 重原子数目 |
195
|
| 分子复杂度/Complexity |
6190
|
| 定义原子立体中心数目 |
26
|
| SMILES |
C[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCNC(=N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(=N)N)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N3CCC[C@H]3C(=O)O)N
|
| InChi Key |
FAWLNURBQMTKEB-URDPEVQOSA-N
|
| InChi Code |
InChI=1S/C129H230N36O29S/c1-64(2)57-87(156-117(183)92(62-69(11)12)158-123(189)100(72(17)18)161-106(172)78(25)143-119(185)94-41-35-54-164(94)126(192)93(63-70(13)14)159-118(184)90(60-67(7)8)154-104(170)76(23)144-121(187)99(71(15)16)160-105(171)77(24)141-102(168)74(21)132)113(179)142-75(22)103(169)153-89(59-66(5)6)116(182)157-88(58-65(3)4)114(180)145-79(26)124(190)163-53-34-42-95(163)120(186)162-101(73(19)20)122(188)151-85(45-47-98(134)167)112(178)149-82(39-32-51-139-128(135)136)108(174)146-80(37-28-30-49-130)107(173)148-83(40-33-52-140-129(137)138)109(175)150-84(44-46-97(133)166)111(177)147-81(38-29-31-50-131)110(176)155-91(61-68(9)10)115(181)152-86(48-56-195-27)125(191)165-55-36-43-96(165)127(193)194/h64-96,99-101H,28-63,130-132H2,1-27H3,(H2,133,166)(H2,134,167)(H,141,168)(H,142,179)(H,143,185)(H,144,187)(H,145,180)(H,146,174)(H,147,177)(H,148,173)(H,149,178)(H,150,175)(H,151,188)(H,152,181)(H,153,169)(H,154,170)(H,155,176)(H,156,183)(H,157,182)(H,158,189)(H,159,184)(H,160,171)(H,161,172)(H,162,186)(H,193,194)(H4,135,136,139)(H4,137,138,140)/t74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,99-,100-,101-/m0/s1
|
| 化学名 |
(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-aminopropanoyl]amino]propanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carbonyl]amino]propanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]propanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]propanoyl]pyrrolidine-2-carbonyl]amino]-3-methylbutanoyl]amino]-5-oxopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-oxopentanoyl]amino]hexanoyl]amino]-4-methylpentanoyl]amino]-4-methylsulfanylbutanoyl]pyrrolidine-2-carboxylic acid
|
| 别名 |
SN50; 213546-53-3; NFkappaB Inhibitor; NF-kappaB Inhibitor, SN50; H-ALA-ALA-VAL-ALA-LEU-LEU-PRO-ALA-VAL-LEU-LEU-ALA-LEU-LEU-ALA-PRO-VAL-GLN-ARG-LYS-ARG-GLN-LYS-LEU-MET-PRO-OH; NF-kappaB SN50; SN50 trifluoroacetate salt; AAVALLPAVLLALLAPVQRKRQKLMP;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~35.95 mM)
H2O : ~50 mg/mL (~17.98 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 33.33 mg/mL (11.98 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.3595 mL | 1.7976 mL | 3.5952 mL | |
| 5 mM | 0.0719 mL | 0.3595 mL | 0.7190 mL | |
| 10 mM | 0.0360 mL | 0.1798 mL | 0.3595 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。