| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
β3-adrenergic receptor (Ki = 0.4 nM for human β3; Ki > 10,000 nM for human β1/β2; EC50 = 1.8 nM for cAMP accumulation in human β3-expressing cells) [1]
β3-adrenergic receptor (high selectivity over β1 and β2 subtypes; no significant binding to α1, α2-adrenergic receptors or other GPCRs) [2] |
|---|---|
| 体外研究 (In Vitro) |
在表达人β3肾上腺素能受体的CHO细胞中,Solabegron呈浓度依赖性诱导cAMP积累(EC50=1.8 nM),且具有完全激动活性(最大反应与异丙肾上腺素相当)。竞争性结合实验显示其对人β3受体亲和力高(Ki=0.4 nM),对β1/β2受体结合可忽略(Ki>10,000 nM)[1]
在分离的犬膀胱逼尿肌条中,Solabegron(1-1000 nM)可使预收缩状态(卡巴胆碱诱导)的肌条呈浓度依赖性松弛。该松弛反应可被β3选择性拮抗剂L-748,337拮抗,证实为β3受体介导的作用[1] 在人及大鼠膀胱平滑肌细胞中,Solabegron(0.1-100 nM)可升高cAMP水平并抑制钙内流,从而降低细胞收缩能力。在心脏或血管平滑肌细胞中未观察到β1/β2受体的显著激活[2] |
| 体内研究 (In Vivo) |
静脉注射 3 mg/kg 的 Solabegron (GW427353) 可促进狗尿量阈值的升高,从而减少乙酸引起的狗尿量减少。低剂量(1 mg/kg)没有表现出任何有意义的作用[1]。
在麻醉雄性比格犬中,静脉注射Solabegron(0.1-10 mg/kg)呈剂量依赖性增加膀胱容量(10 mg/kg时最大增加42%)和排尿反射阈值(10 mg/kg时最大增加58%),且不改变平均动脉压或心率[1] 在乙酸诱导过度活跃膀胱的清醒犬中,口服Solabegron(1-30 mg/kg)可减少排尿频率(30 mg/kg时最大减少35%)并增加单次排尿量(30 mg/kg时最大增加40%)[1] 在部分膀胱出口梗阻诱导的大鼠过度活跃膀胱模型中,口服Solabegron(0.3-10 mg/kg)可改善尿动力学参数:增加膀胱顺应性、减少逼尿肌过度活动、延长排尿间隔。未检测到对心血管功能的显著影响[2] |
| 酶活实验 |
竞争性放射性配体结合实验:将表达人β1/β2/β3肾上腺素能受体的细胞膜与[125I]-氰基吲哚洛尔(放射性配体)及Solabegron(0.01 nM-10 μM)在25°C孵育60分钟。通过过滤分离结合态与游离态配体,测定放射性强度,采用非线性回归计算Ki值[1]
cAMP积累实验:将表达β3受体的CHO细胞接种于96孔板,与Solabegron(0.01 nM-10 μM)在37°C孵育30分钟。裂解细胞后,通过酶免疫法定量cAMP水平,从浓度-反应曲线推导EC50值[1] 受体亚型选择性实验:将表达人β1、β2、α1、α2受体或其他G蛋白偶联受体的细胞用Solabegron(10 μM)处理,检测功能反应(cAMP积累、钙动员),未观察到非靶标受体的显著激活[2] |
| 细胞实验 |
分离犬膀胱逼尿肌细胞并在DMEM/F12培养基中培养。融合细胞经Solabegron(0.1-1000 nM)预处理20分钟后,用卡巴胆碱(1 μM)刺激诱导收缩。通过阻抗测量评估细胞收缩能力,采用ELISA定量cAMP水平[1]
在胶原包被的培养板中培养人膀胱平滑肌细胞,用Solabegron(0.1-100 nM)处理30分钟后,通过荧光钙指示剂(Fura-2 AM)在荧光显微镜下检测钙内流。蛋白质印迹分析证实cAMP依赖性蛋白激酶(PKA)磷酸化水平升高[2] |
| 动物实验 |
Animal/Disease Models: Adult female beagle dog (6-10 kg) [1].
Doses: 1, 3 mg/kg. Doses: IV once. Experimental Results: A dose of 3 mg/kg caused an increase in the urine output threshold. Anesthetized male beagle dogs (8-12 kg) were instrumented with a transurethral bladder catheter and arterial pressure transducer. Solabegron was dissolved in 0.9% saline (intravenous) or 0.5% methylcellulose (oral) and administered at doses of 0.1-10 mg/kg (IV) or 1-30 mg/kg (oral). Bladder pressure, micturition frequency, and cardiovascular parameters were recorded continuously for 4 hours post-administration [1] Conscious dogs with acetic acid-induced overactive bladder: Dogs were acclimated to metabolic cages, and intravesical acetic acid (0.25%) was infused to induce frequent micturition. Solabegron (1-30 mg/kg, oral) was administered, and micturition events, voided volume, and residual urine volume were recorded over 8 hours [1] Male Sprague-Dawley rats (250-300 g) with partial bladder outlet obstruction: Obstruction was induced surgically 2 weeks prior to drug treatment. Solabegron (0.3-10 mg/kg, oral) was given once daily for 7 days. Urodynamic studies were performed under anesthesia to measure bladder compliance, detrusor pressure, and inter-micturition intervals [2] |
| 药代性质 (ADME/PK) |
In beagle dogs, oral administration of Solabegron (10 mg/kg) resulted in peak plasma concentration (Cmax) of 89 ng/mL at 2 hours (Tmax), oral bioavailability of 32%, and elimination half-life (t1/2) of 4.6 hours. Intravenous administration (1 mg/kg) showed a t1/2 of 3.8 hours and volume of distribution (Vd) of 1.2 L/kg [2]
In rats, Solabegron was well-absorbed after oral dosing (bioavailability 45%), with extensive distribution to bladder tissue (tissue/plasma ratio = 2.3 at 1 hour post-dose). Metabolism occurred primarily via glucuronidation, with >70% of the dose excreted in urine as unchanged drug and glucuronide conjugate [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
In repeated-dose toxicity studies (28 days) in rats and dogs, Solabegron (up to 100 mg/kg/day, oral) showed no significant adverse effects on body weight, hematology, or liver/kidney function. No histopathological abnormalities were observed in major organs [2]
Plasma protein binding: Solabegron bound to human plasma proteins at 82±3% (in vitro), with no concentration-dependent binding (0.1-10 μg/mL) [2] No significant cardiovascular toxicity was detected: In dogs, doses up to 30 mg/kg (oral) did not alter heart rate, QTc interval, or mean arterial pressure. No drug-drug interactions with commonly used antimuscarinic agents were observed [1][2] |
| 参考文献 |
|
| 其他信息 |
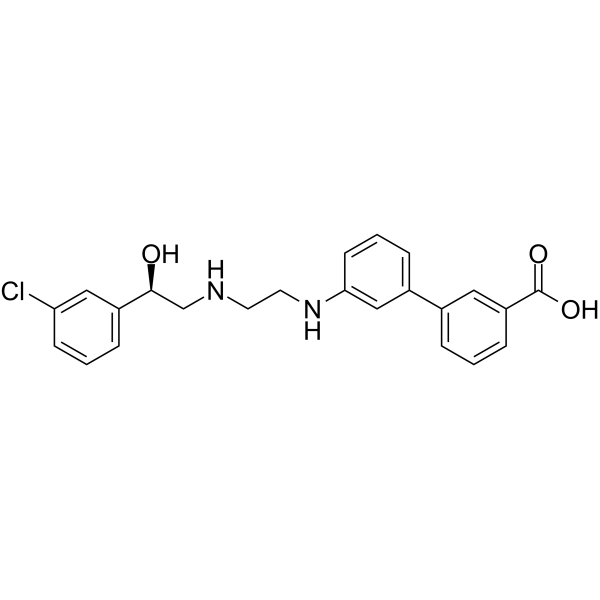
Solabegron is a carboxybiphenyl that is [biphenyl]-3-carboxylic acid carrying a (2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}ethyl)nitrilo group at the 3' position. A selective beta3-adrenergic receptor agonist currently in clinical development for the treatment of overactive bladder and irritable bowel syndrome. It has a role as a beta-adrenergic agonist. It is a secondary alcohol, a substituted aniline, a member of monochlorobenzenes, a secondary amino compound and a carboxybiphenyl.
Solabegron (GW-427,353) is a selective β3 adrenoceptor agonist being developed for the treatment of overactive bladder and irritable bowel syndrome. Drug Indication Investigated for use/treatment in diabetes mellitus type 2, irritable bowel syndrome (IBS), and urinary incontinence. Solabegron is a selective β3-adrenergic receptor agonist that exerts its therapeutic effect by activating β3 receptors in bladder smooth muscle, leading to cAMP accumulation, reduced calcium influx, and bladder relaxation [1][2] The drug is being developed for the treatment of overactive bladder (OAB), targeting symptoms including urinary frequency, urgency, and nocturia [2] Unlike non-selective β-agonists, Solabegron’s high selectivity for β3 receptors avoids β1-mediated cardiac effects and β2-mediated vascular/bronchial effects, improving safety profile [1][2] |
| 分子式 |
C23H23CLN2O3
|
|---|---|
| 分子量 |
410.8933
|
| 精确质量 |
410.139
|
| CAS号 |
252920-94-8
|
| PubChem CID |
9887812
|
| 外观&性状 |
Solid powder
|
| LogP |
1.8
|
| tPSA |
81.6
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
507
|
| 定义原子立体中心数目 |
1
|
| SMILES |
ClC1=C([H])C([H])=C([H])C(=C1[H])[C@]([H])(C([H])([H])N([H])C([H])([H])C([H])([H])N([H])C1=C([H])C([H])=C([H])C(C2C([H])=C([H])C([H])=C(C(=O)O[H])C=2[H])=C1[H])O[H]
|
| InChi Key |
LLDXOPKUNJTIRF-QFIPXVFZSA-N
|
| InChi Code |
InChI=1S/C23H23ClN2O3/c24-20-8-2-6-18(13-20)22(27)15-25-10-11-26-21-9-3-5-17(14-21)16-4-1-7-19(12-16)23(28)29/h1-9,12-14,22,25-27H,10-11,15H2,(H,28,29)/t22-/m0/s1
|
| 化学名 |
3-[3-[2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]ethylamino]phenyl]benzoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 33.33 mg/mL (~81.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.17 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.17 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4337 mL | 12.1687 mL | 24.3374 mL | |
| 5 mM | 0.4867 mL | 2.4337 mL | 4.8675 mL | |
| 10 mM | 0.2434 mL | 1.2169 mL | 2.4337 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。