| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of radiolabelled sorbic acid, ... the total recovery of radioactivity was approx. 100% of the low and high doses. The major route of metabolism of sorbic acid was via expired CO2 with approx. 85% of the admininstered radioactivity being recovered as CO2 within 4-10 hours post administration. From the rate and extent of this metabolism, it may be concluded that sorbic acid is rapidly and quantitatively absorbed in the gastrointestinal tract. Metabolism / Metabolites Metabolism of sorbic acid in rats is identical to that of normally occurring fatty acids. Under normal conditions of intake, sorbic acid is almost completely oxidized to carbon dioxide and water. Traces (0.1% of dose) may be converted by oxidation to trans,trans-muconic acid. 1,4-Dinitro-2-methylpyrrole, a mutagenic product formed by the interaction of two common food additives, sorbic acid and sodium nitrite, was transformed to 1-nitro-2-methyl-4-aminopyrrole by human fecal mixtures and various intestinal bacterial strains. Following oral administration of radiolabelled sorbic acid, ... the total recovery of radioactivity was approx. 100% of the low and high doses. The major route of metabolism of sorbic acid was via expired CO2 with approx. 85% of the administered radioactivity being recovered as CO2 within 4-10 hours p.a. From the rate and extent of this metabolism, it may be concluded that sorbic acid is rapidly and quantitatively absorbed in the gastrointestinal tract. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sorbic acid is white crystalline solid or powder. It is used as intermediate for plasticizers and lubricants. In addition, it is used as preservative and antimicrobial agent for foods, cosmetics, and pharmaceuticals. To improve the characteristics of drying oils. In alkyd type coatings to improve gloss. To improve milling characteristics of cold rubber. HUMAN EXPOSURE AND TOXICITY: Application of 150 mg of sorbic acid to human skin for 1 hr produced severe irritation. An allergic response to sorbic acid (2.5% in petrolatum) reportedly occurred in five of 606 eczema patients suspected of having contact sensitivities, who were tested over a 3-year period. Allergic contact dermatitis from sorbic acid has most frequently been reported after the use of topical medicaments such as corticosteroid creams that contain this preservative. Twenty-five cases of contact allergy were reported to Unguentum Merck, most of which were due to sorbic acid. Sorbic acid can also cause stinging and nonimmunologic contact urticarial reactions. Ocular irritation associated with the use of a hydrogel lens care system containing 0.10% sorbic acid was observed in 15% of 135 patients. Genotoxicity studies with HeLa cells and plasmid DNA did not find either mutagenic or genotoxic activities. ANIMAL STUDIES: A 4 hour semiocclusive application of sorbic acid to the intact skin of three rabbits did not produce erythema nor edema. Sorbic acid was not a primary irritant or sensitizer when applied in 0.1 M concentrations to guinea pig skin. It was characterized as a severe irritant following application of 1 mg to rabbit skin. The proliferation and survival of rabbit corneal epithelial cells in tissue culture were reduced in the presence of 0.1% sorbic acid. No adverse effects were noted in rats fed sorbic acid at dietary levels of 1, 2, 4, and 8% for 90 days. Similarly, there were not adverse findings when sorbic acid was fed to puppies at a 4% dietary level for 90 days. Sorbic acid at dietary levels of 1, 5, or 10% for 80 weeks fed to male and female mice and 0, 1.5, or 10% for 2 years fed to male and female rats did not increase the number of deaths or the incidence of spontaneous histological lesions, including tumors. However, mice fed a diet containing 15% sorbic acid for 88 weeks exhibited a high incidence of hepatoma. The hepatomas that developed in mice fed a 15% sorbic acid diet were considered to be induced both by the chronic depletion of the hepatic glutathione and by the gradual production of various promutagens in the intestine which were absorbed and metabolically activated by the liver. There was no adverse effect on the blood or internal organs of rats, guinea pigs, rabbits, and dogs after prolonged feeding at 1 to 500 times the amount used in foods. In developmental studies in rabbits, no treatment-related maternal or developmental effects were observed at 300 mg/kg bw/day. Maternal findings in the mid dose group included increased respiratory rate following administration, decreased body weight gain and rough surface of the spleen. Maternal findings in high dose females included increased respiratory rate following administration, death, abortion, decreased body weight and body weight gain, marked decrease in food consumption and pathological findings upon necropsy (rough surface and reduced size of the spleen). Statistically significant reductions in mean fetal and placental weights and the viability of the fetuses were observed at the mid and high dose levels. Sorbic acid was inactive in vitro in the Syrian hamster embryo (SHE) fibroblast micronucleus test and the SHE cell transformation test. When administered orally at doses up to 5000 mg/kg, sorbic acid increased the frequency of micronuclei in mice. A significant increase in the frequency of sister chromatid exchanges was observed in bone marrow cells of mice following intraperitoneal injection with 75, 100, or 150 mg/kg of sorbic acid, but not with 25 or 50 mg/kg. When mice were fed a diet containing 15% sorbic acid for a period of up to 6 months, ether extracts of the intestinal contents of the mice were not mutagenic to Salmonella typhimurium TA98, but the acidic components obtained by fractionating the either extracts showed slight mutagenic activity after the addition of a metabolic activation system. These results suggested that mutagens were gradually produced in the intestine and moved into the liver where they were metabolically activated. Sorbic acid was negative in the Salmonella reverse mutation assay (Ames test) with and without metabolic activation. Sorbic acid was also negative in the Chinese hamster fibroblast chromosomal aberration test. Interactions The fungicidal activity of sorbic acid against Saccharomyces cerevisiae was enhanced 64-fold in combination with half-minimum fungicidal concentration of polygodial. This synergistic activity of polygodial presumably comes from its ability to inhibit the plasma membrane H+-ATPase. Sorbic acid has a system of conjugated double bonds which makes it able to undergo nucleophilic addition reactions with certain functions. The interactions between sorbic acid and amine functions present in the endogenous constituents of food were quantified. The formation of new products was demonstrated and the underlying mechanisms studied using ethyl sorbate and various amines. HPLC, GC, GC-SM and NMR analyses of the reaction mixtures enabled the products to be isolated and identified. The addition reactions led, at 20 degrees C, to linear monoadducts and, at 50 degrees C and 80 degrees C, to cyclic derivatives resulting from double addition. Sorbic acid (E200) and its salts (potassium and calcium sorbate: E202 and E203) are allowed for use as preservatives in numerous processed foods. Sorbic acid had a conjugated system of double bonds which makes it susceptible to nucleophilic attack, sometimes giving mutagenic products. Under conditions typical of food processing (50-80 degrees C), we analyzed the cyclic derivatives resulting from a double addition reaction between sorbic acid and various amines. Mutagenesis studies, involving Ames' test and genotoxicity studies with HeLa cells and plasmid DNA, showed that none of the products studied presented either mutagenic or genotoxic activities. The objective of this study was to investigate the occurrence of sublethal injury after the pulsed-electric-field (PEF) treatment of two yeasts, Dekkera bruxellensis and Saccharomyces cerevisiae, as well as the relation of sublethal injury to the inactivating effect of the combination of PEF and sorbic acid. PEF caused sublethal injury in both yeasts: more than 90% of surviving D. bruxellensis cells and 99% of surviving S. cerevisiae cells were sublethally injured after 50 pulses at 12 kV/cm in buffer at pHs of both 7.0 and 4.0. The proportion of sublethally injured cells reached a maximum after 50 pulses at 12.0 kV/cm (S. cerevisiae) or 16.5 kV/cm (D. bruxellensis), and it kept constant or progressively decreased at greater electric field strengths and with longer PEF treatments. Sublethally PEF-injured cells showed sensitivity to the presence of sorbic acid at a concentration of 2,000 ppm. A synergistic inactivating effect of the combination of PEF and sorbic acid was observed. Survivors of the PEF treatment were progressively inactivated in the presence of 2,000 ppm of sorbic acid at pH 3.8, with the combined treatments achieving more than log10 5 cycles of dead cells under the conditions investigated. This study has demonstrated the occurrence of sublethal injury after exposure to PEF, so yeast inactivation by PEF is not an all-or-nothing event. The combination of PEF and sorbic acid has proven to be an effective method to achieve a higher level of yeast inactivation. ... For more Interactions (Complete) data for SORBIC ACID (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat (male) oral >2000 mg/kg bw LD50 Rat (female) oral >2000 mg/kg bw LD50 Rat oral 10,500 mg/kg bw LD50 Rat (male) oral 12,500 mg/kg bw For more Non-Human Toxicity Values (Complete) data for SORBIC ACID (9 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Sorbic acid appears as white powder or crystals. Melting point 134.5 °C. Slightly acidic and astringent taste with a faint odor.
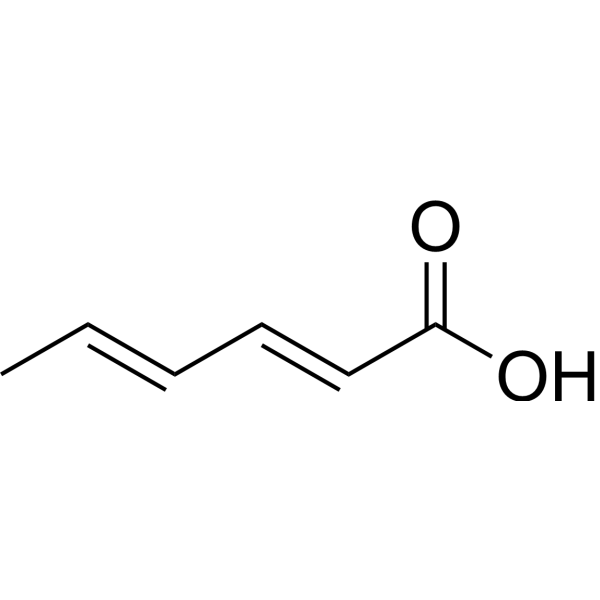
Sorbic acid is a hexadienoic acid with double bonds at C-2 and C-4; it has four geometrical isomers, of which the trans,trans-form is naturally occurring. It is a hexadienoic acid, a polyunsaturated fatty acid, a medium-chain fatty acid and an alpha,beta-unsaturated monocarboxylic acid. It is a conjugate acid of a sorbate. Sorbic acid has been reported in Prunus domestica and Schisandra chinensis with data available. (2E,4E)-2,4-Hexadienoic acid is a preservative for many foodstuffs. Generally used as K salt or (less frequently) as Ca salt. (2E,4E)-2,4-Hexadienoic acid is an antimicrobial agent against a wide variety of microorganisms, especially yeasts and moulds. (2E,4E)-2,4-Hexadienoic acid is a preservative action more efficient in acidic foods. Typical usage levels 500-2000 ppm (2E,4E)-2,4-Hexadienoic acid belongs to the family of Unsaturated Fatty Acids. These are fatty acids whose chain contains at least one CC double bond. Sorbic acid is a metabolite found in or produced by Saccharomyces cerevisiae. Mold and yeast inhibitor. Used as a fungistatic agent for foods, especially cheeses. Therapeutic Uses Food Preservatives The ocular bioavailability of timolol increased in sorbic acid solution due to ion pair formation. Its octanol/water partition coefficient also increased, suggesting the formation of a more lipophilic complex. The concentration of timolol in rabbit aqueous humor was determined after instillation of timolol ophthalmic solution containing sorbic acid. When the molar ratio of sorbic acid to timolol was two or higher, the concentration of timolol in the aqueous humor was higher than with timolol alone. In the presence of sorbic acid the maximal aqueous humor concentration and the area under the curve were more than two-fold higher than those of Timoptol, a timolol maleate ophthalmic solution, and similar in value to TIMOPTIC-XE, a gel-forming ophthalmic solution. To investigate the transcorneal absorption mechanism, in vitro permeation profiles across the intact and de-epithelialyzed cornea were analyzed on the basis of the bilayer diffusion model. The partition coefficient in the epithelium was about twice as high in the presence of sorbic acid than with timolol alone, although the diffusion coefficient in the epithelium did not change. We conclude that the improved ocular bioavailability in the presence of sorbic acid is due to increased partitioning of timolol in the corneal epithelium. Drug Warnings Topical medicaments and cosmetics containing sorbic acid should be avoided. There has been no evidence of flare-ups of eczema from ingestion of foods containing sorbic acid. Therefore, avoiding foods with sorbic acid is unnecessary. |
| 分子式 |
C6H8O2
|
|---|---|
| 分子量 |
112.13
|
| 精确质量 |
112.052
|
| CAS号 |
110-44-1
|
| 相关CAS号 |
Potassium sorbate;24634-61-5
|
| PubChem CID |
643460
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
233.0±9.0 °C at 760 mmHg
|
| 熔点 |
132-135 °C(lit.)
|
| 闪点 |
139.9±9.6 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.488
|
| LogP |
1.35
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
8
|
| 分子复杂度/Complexity |
123
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C/C=C/C=C/C(=O)O
|
| InChi Key |
WSWCOQWTEOXDQX-MQQKCMAXSA-N
|
| InChi Code |
InChI=1S/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+
|
| 化学名 |
(2E,4E)-hexa-2,4-dienoic acid
|
| 别名 |
Hexadienoic acid; Sorbic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~445.91 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (22.30 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (22.30 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (22.30 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.9182 mL | 44.5911 mL | 89.1822 mL | |
| 5 mM | 1.7836 mL | 8.9182 mL | 17.8364 mL | |
| 10 mM | 0.8918 mL | 4.4591 mL | 8.9182 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。