| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Store-operated calcium entry channel Orai
|
|---|---|
| 体外研究 (In Vitro) |
CRAC 通道孔由 Orai 形成,可被 Synta66 抑制。在米勒神经胶质细胞中,Synta66 (10 μM) 降低了峰值 SOCE。在 Trpc1−/− Müller 细胞中,synta66 (10 μM) 会阻止 orai 通道介导残余 SOC 电流 [1]。由注射 CaCl2 引起的 Ca2+ 进入信号几乎被 Synta66 (10 μM) 完全阻断,而血小板中储存的 Ca2+ 的动员仅略微降低 10% 至 30%。 Synta66 (10 μM) 可抑制血浆和全血血栓中的人血小板活化。在小鼠中,Synta66 (10 μM) 还可以预防血栓形成和血小板反应 [2]。 Synta66 (10 μM) 抑制人肥大细胞系 LAD2 的表达。 Synta66 (10 μM) 对人肺肥大细胞 (HLMC) 中 FcεRI 刺激的前列腺素 D2 和细胞因子释放具有不同的影响,并强烈抑制 FcεRI 刺激的组胺和 TNFα 产生 [3]。
|
| 酶活实验 |
SOCE阻断剂抑制血浆和全血血栓形成中的人血小板活化[2]
在血浆或全血系统中,亲脂性抑制剂通常需要以比非血浆缓冲系统高10倍至50倍的浓度添加,以影响血小板功能。28 SOCE抑制剂似乎也是如此。当添加到富含血小板的血浆中时,需要100μmol/L的Synta66、2APB或GSK-7975A的浓度来抑制康夫新诱导的Ca2+升高和41%至49%的PS暴露(数据未显示)。为了验证这些抑制剂是否影响血小板促凝活性,测量了富含血小板血浆中Synta66、2APB或GSK-7975A(100μmol/L)对凝血酶生成的影响。在用1pmol/L组织因子触发后,Synta66、2APB和GSK-7975A的凝血酶生成峰值分别降低到对照组的29±2%、58±2%和28±2% |
| 细胞实验 |
游离[Ca2+]ER降低后,CRAC通道以STIM依赖的方式被激活(Prakriya和Lewis,2015)。为了评估这些Ca2+选择性通道对Müller神经胶质SOCE的贡献,我们在假定的选择性抑制剂Synta66和GSK7975A存在的情况下耗尽了ER储存。Synta66(10μm)将峰值SOCE从511.0±78.5衰减到349.9±40.1nm(N=2;p<0.01),而拮抗剂对基础[Ca2+]i没有显著影响(未处理的对照组为221.5±29.2nm,Synta66处理的细胞为251.7±31.3nm;图5)。同样,野生型细胞中的SOCE反应被GSK-7975A(10μm;图5C-E)部分拮抗。2-APB/SKF 96365/Gd3+消除了Orai抑制细胞中残留的SOCE(图5A,B)。[1]
核心体温激活STIM1,使STIM1与Orai1分离(Xiao等人,2011),并可能刺激Müller细胞中表达的TRPV4热通道(Ryskamp等人,2014)。因此,ICRAC在RT时的激活效果并不理想(Somasundaram等人,1996)。为了确定胶质细胞SOCE是否受温度调节,我们比较了对照组和Synta66处理细胞的过冲反应幅度。从室温到32°C的温度升高导致SOCE适度增加,但没有统计学意义(图5C)。32°C时的SOCE振幅为0.723±0.165,在Synta66存在的情况下降至0.371±0.058(n=6/9个细胞)。尽管减少了约49%,但由于反应的可变性很大,结果没有达到显著性。这些数据表明,Orai与TRPC[Ca2+]SOCE反应的相对分数可能会在核心体温下持续存在。[1] |
| 动物实验 |
Wild-type or chimeric Orai1−/− mice were injected with vehicle solution or 2APB (3 mg/kg) as indicated. In blood samples isolated 60 minutes after injection, collagen-induced thrombus formation was measured, as described above. To induce focal cerebral ischemia in mice, the middle cerebral artery (MCA) was transiently occluded for 60 minutes using an intraluminal filament as described elsewhere (transient MCA occlusion model).20 Immediately after reperfusion of the MCA territory, vehicle solution or 2APB (3 mg/kg) was injected postoperatively. Animals were euthanized on day 1 after transient MCA occlusion, and brain sections were stained with 2% 2,3,5-triphenyltetrazolium chloride to quantify the ischemic brain volume (corrected for edema).[2]
|
| 参考文献 |
|
| 其他信息 |
The endoplasmic reticulum (ER) is at the epicenter of astrocyte Ca(2+) signaling. We sought to identify the molecular mechanism underlying store-operated calcium entry that replenishes ER stores in mouse Müller cells. Store depletion, induced through blockade of sequestration transporters in Ca(2+)-free saline, induced synergistic activation of canonical transient receptor potential 1 (TRPC1) and Orai channels. Store-operated TRPC1 channels were identified by their electrophysiological properties, pharmacological blockers, and ablation of the Trpc1 gene. Ca(2+) release-activated currents (ICRAC) were identified by ion permeability, voltage dependence, and sensitivity to selective Orai antagonists Synta66 and GSK7975A. Depletion-evoked calcium influx was initiated at the Müller end-foot and apical process, triggering centrifugal propagation of Ca(2+) waves into the cell body. EM analysis of the end-foot compartment showed high-density ER cisternae that shadow retinal ganglion cell (RGC) somata and axons, protoplasmic astrocytes, vascular endothelial cells, and ER-mitochondrial contacts at the vitreal surface of the end-foot. The mouse retina expresses transcripts encoding both Stim and all known Orai genes; Müller glia predominantly express stromal interacting molecule 1 (STIM1), whereas STIM2 is mainly confined to the outer plexiform and RGC layers. Elimination of TRPC1 facilitated Müller gliosis induced by the elevation of intraocular pressure, suggesting that TRPC channels might play a neuroprotective role during mechanical stress. By characterizing the properties of store-operated signaling pathways in Müller cells, these studies expand the current knowledge about the functional roles these cells play in retinal physiology and pathology while also providing further evidence for the complexity of calcium signaling mechanisms in CNS astroglia.[1]
Objective: Platelet Orai1 channels mediate store-operated Ca(2+) entry (SOCE), which is required for procoagulant activity and arterial thrombus formation. Pharmacological blockage of these channels may provide a novel way of antithrombotic therapy. Therefore, the thromboprotective effect of SOCE blockers directed against platelet Orai1 is determined. Methods and results: Candidate inhibitors were screened for their effects on SOCE in washed human platelets. Tested antagonists included the known compounds, SKF96365, 2-aminoethyl diphenylborate, and MRS1845 and the novel compounds, Synta66 and GSK-7975A. The potency of SOCE inhibition was in the order of Synta66>2-aminoethyl diphenylborate>GSK-7975A>SKF96365>MRS1845. The specificity of the first 3 compounds was verified with platelets from Orai1-deficient mice. Inhibitory activity on procoagulant activity and high-shear thrombus formation was assessed in plasma and whole blood. In the presence of plasma, all 3 compounds suppressed platelet responses and restrained thrombus formation under flow. Using a murine stroke model, arterial thrombus formation was provoked in vivo by transient middle cerebral artery occlusion. Postoperative administration of 2-aminoethyl diphenylborate markedly diminished brain infarct size. Conclusions: Plasma-soluble SOCE blockers such as 2-aminoethyl diphenylborate suppress platelet-dependent coagulation and thrombus formation. The platelet Orai1 channel is a novel target for preventing thrombotic events causing brain infarction.[2] Inappropriate activation of mast cells via the FcεRI receptor leads to the release of inflammatory mediators and symptoms of allergic disease. Calcium influx is a critical regulator of mast cell signaling and is required for exocytosis of preformed mediators and for synthesis of eicosanoids, cytokines and chemokines. Studies in rodent and human mast cells have identified Orai calcium channels as key contributors to FcεRI-initiated mediator release. However, until now the role of TRPC calcium channels in FcεRI-mediated human mast cell signaling has not been published. Here, we show evidence for the expression of Orai 1,2, and 3 and TRPC1 and 6 in primary human lung mast cells and the LAD2 human mast cell line but, we only find evidence of functional contribution of Orai and not TRPC channels to FcεRI-mediated calcium entry. Calcium imaging experiments, utilizing an Orai selective antagonist (Synta66) showed the contribution of Orai to FcεRI-mediated signaling in human mast cells. Although, the use of a TRPC3/6 selective antagonist and agonist (GSK-3503A and GSK-2934A, respectively) did not reveal evidence for TRPC6 contribution to FcεRI-mediated calcium signaling in human mast cells. Similarly, inactivation of STIM1-regulated TRPC1 in human mast cells (as tested by transfecting cells with STIM1-KK684-685EE - TRPC1 gating mutant) failed to alter FcεRI-mediated calcium signaling in LAD2 human mast cells. Mediator release assays confirm that FcεRI-mediated calcium influx through Orai is necessary for histamine and TNFα release but is differentially involved in the generation of cytokines and eicosanoids.[3] |
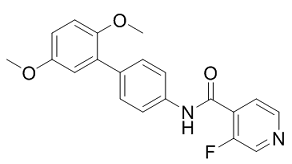
| 分子式 |
C20H17N2O3F
|
|---|---|
| 分子量 |
352.359
|
| 精确质量 |
352.122
|
| CAS号 |
835904-51-3
|
| PubChem CID |
11337104
|
| 外观&性状 |
Typically exists as White to gray solids at room temperature
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
422.4±45.0 °C at 760 mmHg
|
| 闪点 |
209.3±28.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.611
|
| LogP |
2.52
|
| tPSA |
60.4Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
456
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1C(F)=CN=CC=1)NC1C=CC(C2C(OC)=CC=C(OC)C=2)=CC=1
|
| InChi Key |
GFEIWXNLDKUWIK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H17FN2O3/c1-25-15-7-8-19(26-2)17(11-15)13-3-5-14(6-4-13)23-20(24)16-9-10-22-12-18(16)21/h3-12H,1-2H3,(H,23,24)
|
| 化学名 |
N-[4-(2,5-dimethoxyphenyl)phenyl]-3-fluoropyridine-4-carboxamide
|
| 别名 |
Synta-66; CHEMBL3403742; SCHEMBL1829334; CHEBI:231608; GLXC-03244; GSK1349571A;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~77.5 mg/mL (~219.95 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.58 mg/mL (7.32 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声和加热处理
例如,若需制备1 mL的工作液,可将100 μL 25.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.58 mg/mL (7.32 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 25.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.58 mg/mL (7.32 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8380 mL | 14.1900 mL | 28.3801 mL | |
| 5 mM | 0.5676 mL | 2.8380 mL | 5.6760 mL | |
| 10 mM | 0.2838 mL | 1.4190 mL | 2.8380 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。