| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g | |||
| Other Sizes |
| 靶点 |
ER/Estrogen receptor; HSP90
|
|---|---|
| 体外研究 (In Vitro) |
Tamoxifen (ICI 47699) 不影响 MDA-MB-231 细胞,但对 MCF-7 细胞有显着的抑制作用 (EC50=1.41 μM),对 T47D 细胞的抑制作用较弱 (EC50=2.5 μM) [2] 。
|
| 体内研究 (In Vivo) |
当预突变小鼠接受注射他莫昔芬(75 mg/kg;在 6 周龄每五天注射一次)时,就会发生基因敲除,导致 floxed 外显子切除 [3]。
他莫昔芬诱导型基因敲除策略具有明显的优势,因为可以以组织特异性的方式在成年小鼠中随意消除基因的表达。为了研究Med1在成年心脏中的作用,7周龄的TmcsMed1-/-小鼠每天以65mg/kg的剂量腹腔注射他莫昔芬5天,然后在选定的时间间隔内处死。RNA的qPCR分析显示,注射他莫昔芬3天后,Med1表达开始下降(约下降70%),注射5天后,心脏中几乎检测不到Med1表达。在成年小鼠中,他莫昔芬诱导的Med1心脏特异性破坏(TmcsMed1-/-)会导致扩张型心肌病[8]。 |
| 酶活实验 |
他莫昔芬是一种选择性雌激素受体调节剂,广泛用于预防性治疗癌症。除了雌激素受体(ER)外,他莫昔芬还与微粒体抗雌激素结合位点(AEBS)具有高亲和力,AEBS参与了他莫昔芬的ER非依赖性作用。在本研究中,我们研究了AEBS配体对肿瘤细胞系中胆固醇生物合成的调节。作为抗肿瘤药物他莫昔芬或PBPE(一种选择性AEBS配体)治疗的结果,我们发现肿瘤细胞产生了显著的胆固醇前体浓度和时间依赖性积聚。甾醇已通过HPLC和气相色谱法纯化,其化学结构通过质谱分析确定。鉴定的主要代谢产物是用于他莫昔芬治疗的5α-胆固醇-8-en-3β醇和用于PBPE治疗的5β-胆固醇-8en-3β醇和胆固醇-5,7-二烯-3β醇,这表明这些AEBS配体至少影响两个酶步骤:3β-羟基甾醇-Deta8-Delta7-异构酶和3β-羟甾醇-Deta7-还原酶。类固醇抗雌激素如ICI 182780和RU 58668不影响这些酶促步骤,因为它们不与AEBS结合。人3-β-羟基甾醇-Deta8-Delta7-异构酶和3-β-羟甾醇-Deta7-还原酶的瞬时共表达和免疫沉淀实验表明,在哺乳动物细胞中重建AEBS需要这两种酶。总之,这些数据提供了强有力的证据,表明AEBS是一种异源寡聚复合物,包括作为他莫昔芬在乳腺细胞中结合所必需和充分的亚基的3-β-羟基甾醇-Deta8-Delta7-异构酶和3-β-羟甾醇-Deta7-还原酶。此外,由于选择性AEBS配体是抗肿瘤化合物,这些数据表明羊毛甾醇后步骤的胆固醇代谢与肿瘤生长控制之间存在联系。这些数据既提供了AEBS的鉴定,又为具有临床价值的药物的新分子作用机制提供了新的见解[5]。
|
| 细胞实验 |
先前的研究表明,来自当地热带植物的苯乙烯基吡咯酮衍生物(SPD)在早孕小鼠模型中具有抗孕激素和抗雌激素作用(Azimahtol等人,1991)。抗孕激素和抗雌激素可作为治疗癌症的一种治疗方法,因此在三种不同的人癌症细胞系MCF-7、T47D和MDA-MB-231中测试了SPD的抗肿瘤活性,采用Lin和Hwang(1991)的抗增殖试验进行了微改性。SPD(10(-10)-10(-6)M)在雌激素和孕激素依赖性MCF-7细胞(EC50=2.24 x 10(-7)M)和激素不敏感的MDA-MB-231(EC50=5.62 x 10(-7M))中表现出很强的抗增殖活性,但仅对雌激素不敏感的T47D细胞(EC50=1.58 x 10(-6)M)产生部分抑制作用。然而,他莫昔芬对MCF-7细胞显示出强烈的抑制作用(EC50=1.41 x 10(-6)M),对T47D细胞的抑制作用较小(EC50=2.5 x 10(-6M)M)但对MDA-MB-231细胞没有影响。1微M的SPD在抑制1 nM雌二醇刺激的MCF-7细胞生长方面比1微M他莫昔芬具有更强的抗雌激素活性。SPD和他莫昔芬在1微M下的联合治疗对培养的MCF-7细胞的生长显示出额外的抑制作用。SPD的抗增殖特性对受体阳性和受体阴性的乳腺癌症细胞都有效,因此似乎既不依赖于细胞受体状态,也不取决于细胞激素反应。这增强了体内方法,因为肿瘤是具有不同受体状态的异质性肿块[2]。
|
| 动物实验 |
Animal/Disease Models: Aldh1l1-cre/ERT2 x Ai95 mice[3]
Doses: 75 mg/kg Route of Administration: Injected for 5 days at 6 weeks of age Experimental Results: Resulted in the excision of the floxed exon and a gene knockout. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
An oral dose of 20mg reaches a Cmax of 40ng/mL with a Tmax of 5 hours. The metabolite N-desmethyltamoxifen reaches a Cmax of 15ng/mL. 10mg of tamoxifen orally twice daily for 3 months results in a Css of 120ng/mL and a Css of 336ng/mL. Tamoxifen is mainly eliminated in the feces. Animal studies have shown 75% of radiolabelled tamoxifen recovered in the feces, with negligible collection from urine. However, 1 human study showed 26.7% recovery in the urine and 24.7% in the feces. The volume of distribution of tamoxifen is approximately 50-60L/kg. The clearance of tamoxifen was 189mL/min in a study of six postmenopausal women. Tamoxifen appears to be absorbed slowly following oral administration, with peak serum concentrations generally occurring about 3-6 hours after a single dose. The extent of absorption in humans has not been adequately determined, but limited data from animal studies suggest that the drug is well absorbed. Data from animal studies also suggest that tamoxifen and/or its metabolites undergo extensive enterohepatic circulation. Following oral administration, peak serum tamoxifen concentrations average about 17 ng/mL after a single 10-mg dose, about 40 ng/mL after a single 20-mg dose, and 65-70 ng/mL after a single 40-mg dose; however, there is considerable interindividual variation in serum tamoxifen concentrations attained after single doses and at steady state with continuous dosing. Following a single oral dose of tamoxifen, peak serum concentrations of N-desmethyltamoxifen, the major metabolite of the drug, generally range from about 15-50% those of unchanged tamoxifen; however, with continuous dosing, steady-state serum concentrations of N-desmethyltamoxifen generally range from about 1-2 times those of unchanged tamoxifen. Following continuous administration in patients receiving oral tamoxifen 10 mg twice daily for 3 months, steady-state plasma concentrations of tamoxifen and N-desmethyltamoxifen average about 120 ng/mL (range: 67-183 ng/mL) and 336 ng/mL (range: 148-654 ng/mL), respectively. Steady-state serum concentrations of tamoxifen are generally attained after 3-4 weeks of continuous dosing, while those of N-desmethyltamoxifen are generally attained after 3-8 weeks of continuous dosing. Steady-state serum concentrations can be attained more rapidly with a loading-dose regimen, but there is no therapeutic advantage with such a regimen. For more Absorption, Distribution and Excretion (Complete) data for TAMOXIFEN (9 total), please visit the HSDB record page. Metabolism / Metabolites Tamoxifen can by hydroxylated to α-hydroxytamoxifen which is then glucuronidated or undergoes sulfate conjugation by sulfotransferase 2A1. Tamoxifen can also undergo N-oxidation by flavin monooxygenases 1 and 3 to tamoxifen N-oxide. Tamoxifen is N-dealkylated to N-desmethyltamoxifen by CYP2D6, CYP1A1, CYP1A2, CYP3A4, CYP1B1, CYP2C9, CYP2C19, and CYP3A5. N-desmethyltamoxifen can be sulfate conjugated to form N-desmethyltamoxifen sulfate, 4-hydroxylated by CYP2D6 to form endoxifen, or N-dealkylated again by CYP3A4 and CYP3A5 to N,N-didesmethyltamoxifen. N,N-didesmethyltamoxifen undergoes a substitution reaction to form tamoxifen metabolite Y, followed by ether cleavage to metabolite E, which can then be sulfate conjugated by sulfotransferase 1A1 and 1E1 or O-glucuronidated. Tamoxifen can also by 4-hydroxylated by CYP2D6, CYP2B6, CYP3A4, CYP2C9, and CYP2C19 to form 4-hydroxytamoxifen. 4-hydroxytamoxifen can undergo glucuronidation by UGT1A8, UGT1A10, UGT2B7, and UGT2B17 to tamoxifen glucuronides, sulfate conjugation by sulfotransferase 1A1 and 1E1 to 4-hydroxytamoxifen sulfate, or N-dealkylation by CYP3A4 and CYP3A5 to endoxifen. Endoxifen undergoes demethylation to norendoxifen, a reversible sulfate conjugation reaction via sulfotransferase 1A1 and 1E1 to 4-hydroxytamoxifen sulfate, sulfate conjugation via sulfotransferase 2A1 to 4-endoxifen sulfate, or glucuronidation via UGT1A8, UGT1A10, UGT2B7, or UGT2B15 to tamoxifen glucuronides. Tamoxifen is extensively metabolized after oral administration. N-desmethyl tamoxifen is the major metabolite found in plasma. N-desmethyl tamoxifen activity is similar to tamoxifen. 4-Hydroxytamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. Tamoxifen is a substrate of cytochrome P450 CYP3A, CYP2C9 and CYP2D6, and an inhibitor of P-glycoprotein. Tamoxifen is rapidly and extensively metabolized, principally by demethylation and to a small degree by subsequent deamination and also by hydroxylation. Initial studies suggested that 4-hydroxytamoxifen (metabolite B) was the major metabolite of the drug, but subsequent studies using improved assay methodologies have shown that 4-hydroxytamoxifen is a minor metabolite and that the major metabolite is N-desmethyltamoxifen (metabolite X). The biologic activity of N-desmethyltamoxifen appears to be similar to that of tamoxifen. N-Desmethyltamoxifen undergoes demethylation to form N,N-desdimethyltamoxifen (metabolite Z) which undergoes subsequent deamination to form the primary alcohol metabolite (metabolite Y). Both 4-hydroxytamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. 3,4-Dihydroxytamoxifen and an unidentified metabolite (metabolite E) also have been detected in plasma in small amounts. With continuous administration of tamoxifen, serum concentrations of N-desmethyltamoxifen are generally about 1-2 times those of unchanged tamoxifen, while those of N,N-desdimethyltamoxifen are about 20-40% those of unchanged tamoxifen and those of the primary alcohol metabolite are about 5-25% those of unchanged tamoxifen; concentrations of the hydroxylated metabolites and metabolite E appear to be less than 5% of those of unchanged tamoxifen. Several metabolites of tamoxifen, including 4-hydroxy-N-desmethyltamoxifen, 4-hydroxytamoxifen, N-desmethyltamoxifen, the primary alcohol, and N-desdimethyltamoxifen were identified and their concn determined in fluids and feces from patients receiving chronic tamoxifen treatment. The biological samples investigated were serum, pleural, pericardial and peritoneal effusions, cerebrospinal fluid, saliva, bile, feces, and urine. In serum, tamoxifen itself, and the metabolites N-desmethyltamoxifen and N-desdimethyltamoxifen were the prevailing species, but significant amounts of the metabolites the primary alcohol, 4-hydroxytamoxifen, 4-hydroxy-N-desmethyltamoxifen were also detected. About 3 hr after drug intake tamoxifen as well as, N-desmethyltamoxifen, an N-desdimethyltamoxifen) showed a peak in serum. This may be explained by efficient metabolism of the metabolite precursor before being distributed to peripheral compartments. Upon drug withdrawal all metabolites showed first-order elimination curves which paralleled that of tamoxifen suggesting that their rate of elimination exceeded that of tamoxifen and that the serum levels are production rate limited. The protein binding of tamoxifen and its major serum metabolites (the primary alcohol, N-desmethyltamoxifen, N-desdimethyltamoxifen) was determined and found to be higher than 98%. Albumin was the predominant carrier for tamoxifen in human plasma. The concn of tamoxifen and its metabolites in pleural, pericardial, and peritoneal effusions equalled those detected in serum, corresponding to an effusion/serum ratio between 0.2 and 1. Only trace amounts of tamoxifen and metabolite N-desmethyltamoxifen were detected in cerebrospinal fluid (CSF/serum ratio less than 0.02). In saliva, concn of tamoxifen and N-desmethyltamoxifen exceeded the amounts of free drug in serum, suggesting active transport or trapping of these compounds in the salivary gland. Bile and urine were rich in the hydroxylated, conjugated metabolites (the primary alcohol, 4-hydroxytamoxifen, 4-hydroxy-N-desmethyltamoxifen, whereas in feces unconjugated metabolite B and tamoxifen were the predominating species. The amount of tamoxifen, N-desmethyltamoxifen (metabolite X), N-desdimethyltamoxifen (metabolite Z), and hydroxylated metabolites (trans-1(4-beta-hydroxyethoxyphenyl)-1,2-diphenylbut-1-ene, 4-hydroxytamoxifen and 4-hydroxy-N-desmethyltamoxifen) were determined in brain metastases from breast cancer patients and in the surrounding brain tissues. Specimens were collected from the breast cancer patients who received tamoxifen for 7-180 days and with the last dose taken within 28 hr before surgical removal of the tumour. The concn of tamoxifen and its metabolites were up to 46 fold higher in the brain metastatic tumour and brain tissue than in serum. Metabolite N-desmethyltamoxifen was the most abundant species followed by tamoxifen and metabolite N-desdimethyltamoxifen. Small but significant amounts of the hydroxylated metabolites, trans-1(4-beta-hydroxyethoxyphenyl)-1,2-diphenylbut-1-ene, 4-hydroxytamoxifen and 4-hydroxy-N-desmethyltamoxifen were detected in most specimens. The ratios between the concn of tamoxifen and various metabolites were similar in tumour, brain and serum. This is the first report on the distribution of tamoxifen and metabolites into human brain and brain tumour, and the data form a basis for further investigation into the therapeutic effects of tamoxifen on brain metastases from breast cancer. Tamoxifen has known human metabolites that include 4'-Hydroxytamoxifen, alpha-Hydroxytamoxifen, 3-Hydroxytamoxifen, N-Desmethyltamoxifen, 4-Hydroxytamoxifen, and Tamoxifen N-glucuronide. Hepatic. Tamoxifen is extensively metabolized after oral administration. N-Desmethyl-tamoxifen is the major metabolite found in plasma. N-Desmethyl-tamoxifen's activity is similar to tamoxifen. 4-hydroxy-tamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. 4-Hydroxy-tamoxifen formation is catalyzed mainly by cytochrome P450 (CYP) 2D6, and also by CYP2C9 and 3A4. At high tamoxifen concentrations, CYP2B6 also catalyzes 4-hydroxylation of the parent drug. 4-Hydroxy-tamoxifen possesses 30- to 100-times greater affinity for the estrogen receptor and 30- to 100-times greater potency at inhibiting estrogen-dependent cell proliferation compared to tamoxifen. It is also metabolized by flavin monooxygenases FMO1 and FMO3 to form tamoxifen-N-oxide. Route of Elimination: 65% of the dose was excreted from the body over 2 weeks in which fecal excretion was the primary route of elimination. Tamoxifen is excreted mainly as polar conjugates, with unchanged drug and unconjugated metabolites accounting for less than 30% of the total fecal radioactivity. Half Life: The decline in tamoxifen plasma concentrations is biphasic with a terminal elimination half-life of approximately 5 to 7 days. The estimated half-life of N-desmethyl tamoxifen is 14 days. Biological Half-Life The terminal elimination half-life of tamoxifen is 5 to 7 days, while the half-life of N-desmethyltamoxifen, the primary circulating metabolite, is approximately 14 days. Limited data suggest that tamoxifen has a distribution half-life of 7-14 hours and an elimination half-life of about 5-7 days (range: 3-21 days). The elimination half-life of N-desmethyltamoxifen, the major metabolite, is estimated to be 9-14 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Tamoxifen is an anti-estrogenic non-steroidal drug. Indications: Treatment of advanced breast cancer and adjuvant treatment of early breast cancer. Treatment of anovulatory infertility. HUMAN EXPOSURE: Main risks and target organs: Adverse effects in therapeutic use are usually mild. They include effects caused by antagonism of endogenous oestrogens: hot flushes, non-specific gastrointestinal effects (nausea and vomiting), central nervous system effects, and rare ocular effects. Adverse hematological effects have been reported, also isolated cases of death from peliosis hepatis and from hyperlipidemia. In the treatment of breast cancer, hypercalcemia and tumor flare can occur. Summary of clinical effects: Anti-estrogenic effects in women treated with tamoxifen include vasomotor symptoms (hot flushes), vaginal bleeding and (in premenopausal women) irregular menses, and pruritus vulvae. Nausea and vomiting can occur. Dizziness, lethargy, depression, irritability and cerebellar dysfunction have been described. Reversible retinopathy with macular edema has been reported after high cumulative doses, and corneal changes can occur. Thrombocytopenia or leukopenia have been associated with tamoxifen treatment. Thromboembolism, which may be due to the disease rather than the treatment, has been recorded in women given tamoxifen for breast cancer. Contraindications: Pregnancy is an absolute contraindication because of the anti-estrogenic effects. Routes of entry: Oral: Usual route of entry Absorption by route of exposure: Peak concentrations occur 4-7 hr after oral dosing. Peak concentrations after single oral dose are about 40 u/l. Distribution by route of exposure: Tamoxifen is more than 99% protein-bound in serum, predominantly to albumin. In patients with breast cancer, concentrations of tamoxifen and its metabolites in pleural, pericardial and peritoneal effusion fluid are between 20 and 100% of those in serum, but only trace amounts enter the cerebrospinal fluid. Concentrations in breast cancer tissue exceed those in serum. The volume of distribution is 50-60 l/kg. Biological half-life by route of exposure: The elimination is biphasic, with an initial half-life of around 7 hr and a terminal half-life of 7-11 days. Metabolism: Tamoxifen citrate undergoes extensive hepatic metabolism to: 1-(4-ethanolyloxyphenyl)-1,2-diphenylbut-1-ene (the primary alcohol), N-desmethyl tamoxifen, 4-hydroxy tamoxifen, 4-hydroxy-N-desmethyl tamoxifenn and N-desdimethyl tamoxifen Elimination by route of exposure: The major excretory route is via the bile as metabolites and enterohepatic recirculation occurs. Less than 1% is excreted in the urine. Mode of action: Toxicodynamics: The adverse effects observed are due mainly to its anti-estrogen effect, as Tamoxifen and certain of its metabolites antagonise the effects of estrogens in estrogen sensitive tissues. Pharmacodynamics: Tamoxifen and several of its metabolites (particularly 4-hydroxytamoxifen) bind to nuclear estrogen receptors in estrogen sensitive tissues, and also to a microsomal protein termed the anti-estrogen binding site. Tamoxifen interferes with the physiological sequence by which estrogen binds to its receptor, is translocated in the nucleus and then activates messenger RNA synthesis. Although the tamoxifen receptor complex is transported in the nucleus in the same way as estrogen receptor complex, it fails to activate synthesis of mRNA. Carcinogenicity: A case-control study showed a significantly increased relative risk of carcinoma of the uterus in women previously treated with tamoxifen and who had previously had radiotherapy involving the uterus. The study showed an increase in relative risk with tamoxifen treatment alone which was not statistically significant. Teratogenicity: Studies in neonatal male and female mice at relative doses 10 times higher than those used in humans have shown genital tract abnormalities. Interactions: Tamoxifen potentiates the anticoagulant effect of warfarin, and this interaction can be life-threatening. Main adverse effects: Adverse effects are usually mild. Thrombocytopenia, leukopenia, thromboembolism, peliosis hepatis and hyperlipidaemia have been mentioned in case reports. Severe hypercalcemia can occur rarely when treatment is started in patients with metastases to bone. Chronic poisoning: Ingestion: Retinal damage and keratitis have been reported in patients after large cumulative doses of tamoxifen, for more than 1 year, though sometimes with smaller doses. There seems to be correlation between long term tamoxifen administration and endometrial proliferation. Neurological: CNS: A case of depression, syncope, and incoordination has been described during therapy with 10 mg twice daily. The symptoms resolved when tamoxifen was discontinued and reappeared when treatment was restarted. Gastrointestinal: Nausea and vomiting occur with therapeutic doses in some patients, and are anticipated in overdosage. Hepatic: A fatal case of peliosis hepatis has been reported in a woman treated with tamoxifen for 2 years after mastectomy for carcinoma. Urinary: Other: A case of persistent nocturnal priapism has been reported. Endocrine and reproductive systems: The anti-estrogenic effects of tamoxifen in premenopausal women receiving therapeutic doses can cause irregular menses. Anti-estrogenic adverse effects in women treated with tamoxifen include vasomotor symptoms and vaginal bleeding and pruritus vulvae. Eye, ear, nose, throat: local effects: Treatment has been associated with retinal and corneal changes. Hematological: Thromboembolism may be more common in patients treated with tamoxifen, though this is not certain, as patients with cancer are at increased risk anyway. A small reduction in antithrombin III concentration was noted in a study of 11 postmenopausal women treated with tamoxifen, but it was clinically insignificant, and no significant reduction was seen in a group of premenopausal women. Thrombocytopenia and leukopenia can occur during therapy, but are not usually severe. One case of severe myelosuppression has been reported. Fluid and electrolyte disturbances: Severe hypercalcemia, associated with increased bone resorption, has been noted when patients with bony metastases commenced therapy. Others: Severe hyperlipidemia is occasionally seen, and has been ascribed to an estrogenic effect. Special risks: Pregnancy, breast feeding and enzyme deficiencies. ANIMAL/PLANT STUDIES: In some animal species, estrogenic agonist effects become manifest at dosages equivalent to 10-100 times the human therapeutic dose. Mutagenicity: Tamoxifen is believed not to be mutagenic. /Tamoxifen citrate/ Tamoxifen is a nonsteroidal agent that binds to estrogen receptors (ER), inducing a conformational change in the receptor. This results in a blockage or change in the expression of estrogen dependent genes. The prolonged binding of tamoxifen to the nuclear chromatin of these results in reduced DNA polymerase activity, impaired thymidine utilization, blockade of estradiol uptake, and decreased estrogen response. It is likely that tamoxifen interacts with other coactivators or corepressors in the tissue and binds with different estrogen receptors, ER-alpha or ER-beta, producing both estrogenic and antiestrogenic effects. Interactions Concomitant use of Aminoglutethimide may decrease plasma concentrations of tamoxifen and N-desmethyl tamoxifen. Concomitant use coumarin-derivatived anticoagulants, may result in significant increase in anticoagulant effect; use is contraindicated in women for reducing the risk of breast cancer in high-risk women and women with ductal carcinoma in situ (DCIS). Concomitant use of bromocriptine may increase serum levels of tamoxifen and N-desmethyl tamoxifen. Concomitant use of cytotoxic agents may result in increased risk of thromboembolic events. For more Interactions (Complete) data for TAMOXIFEN (12 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609-18.
[2]. Hawariah A, et al. In vitro response of human breast cancer cell lines to the growth-inhibitory effects of styrylpyrone derivative (SPD) and assessment of its antiestrogenicity. Anticancer Res. 1998 Nov-Dec;18(6A):4383-6. [3]. Jun Nagai, et al. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell. 2019 May 16;177(5):1280-1292.e20. [4]. Zhao R, et al. Tamoxifen enhances the Hsp90 molecular chaperone ATPase activity. PLoS One. 2010 Apr 1;5(4):e9934. [5]. Kedjouar B, et al. Molecular characterization of the microsomal tamoxifen binding site. J Biol Chem. 2004 Aug 6;279(32):34048-61. [6]. Feil S, et, al. Inducible Cre mice. Methods Mol Biol. 2009;530:343-63. [7]. Laura Cooper, et al. Screening and Reverse-Engineering of Estrogen Receptor Ligands as Potent Pan-Filovirus Inhibitors. J Med Chem. 2020 Sep 4. [8]. Cardiomyocyte-Specific Ablation of Med1 Subunit of the Mediator Complex Causes Lethal DilatedCardiomyopathy in Mice. PLoS One. 2016 Aug 22;11(8):e0160755. |
| 其他信息 |
Therapeutic Uses
Anticarcinogenic Agents; Antineoplastic Agents, Hormonal; Carcinogens; Estrogen Antagonists Antiestrogen; antineoplastic (hormonal). Tamoxifen is indicated for adjuvant treatment of axillary nodenegative breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation. Data are insufficient to predict which women are most likely to benefit and to determine if tamoxifen provides any benefit in women with tumors of less than 1 cm. Tamoxifen is /also/ indicated for adjuvant treatment of axillary node-positive breast cancer in postmenopausal women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation. In some tamoxifen adjuvant studies, most of the benefit to date has been in the subgroup with four or more positive axillary nodes. /Include in US product label/ Tamoxifen is indicated to reduce the risk of developing breast cancer in women who have been determined to be at high risk for developing this cancer. A woman is considered to be at high risk if she is at least 35 years of age and has a 5-year predicted risk of developing breast cancer greater than or equal to 1.67%. /Included in US product label/ For more Therapeutic Uses (Complete) data for TAMOXIFEN (8 total), please visit the HSDB record page. Drug Warnings Cases of tamoxifen-associated hepatotoxicity have been described, including cholestasis with or without cytolysis and steatohepatitis. We report the case of a female patient who developed hepatic alterations while undergoing continuous tamoxifen treatment. There are recent reports of postmenopausal bleeding from endometrial polyps in women receiving tamoxifen therapy for breast cancer. /The authors/ describe four additional patients who presented with vaginal bleeding, and emphasize the pathology. These polyps demonstrated cystically dilated glands in all cases and stromal decidualization in two; in one instance, metastatic breast carcinoma was present in the polyp. The mechanisms by which tamoxifen may affect the development of these polyps are discussed. This case report serves to emphasize two important features of metastatic breast carcinoma. First, that tamoxifen-induced flare, although a rare and self-limiting phenomenon, may be fatal and must thus be recognized and treated promptly. Secondly, those patients presenting with hypercalcaemia, as part of tamoxifen-induced tumour flare, invariably have metastatic disease but they may enjoy a durable prognosis if this is confined to the skeleton. The fourth case of heterologous mesodermal tumour of the uterine corpus, that developed, years following tamoxifen therapy for breast cancer in a postmenopausal woman with no previous pelvic irradiation, is presented with coincidental endometriosis and endometrial intraepithelial carcinoma. For more Drug Warnings (Complete) data for TAMOXIFEN (42 total), please visit the HSDB record page. Pharmacodynamics Tamoxifen is a selective estrogen receptor modulator that inhibits growth and promotes apoptosis in estrogen receptor positive tumors. It has a long duration of action as the active metabolite N-desmethyltamoxifen has a half life of approximately 2 weeks. It has a narrow therapeutic index as higher doses can lead to breathing difficulty or convulsions. Tamoxifen administration is also associated with an increased incidence of uterine malignancies. |
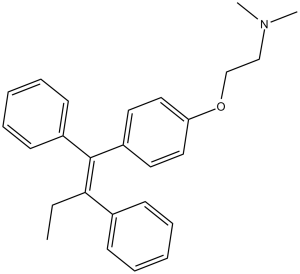
| 分子式 |
C26H29NO
|
|---|---|
| 分子量 |
371.51
|
| 精确质量 |
371.224
|
| 元素分析 |
C, 84.06; H, 7.87; N, 3.77; O, 4.31
|
| CAS号 |
10540-29-1
|
| 相关CAS号 |
Tamoxifen Citrate;54965-24-1;Tamoxifen (Standard);10540-29-1;Tamoxifen-d5;157698-32-3;Tamoxifen-d3;508201-30-7
|
| PubChem CID |
2733526
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
482.3±33.0 °C at 760 mmHg
|
| 熔点 |
97-98ºC
|
| 闪点 |
140.0±27.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.582
|
| LogP |
7.88
|
| tPSA |
12.47
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
463
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])/C(/C1C([H])=C([H])C([H])=C([H])C=1[H])=C(\C1C([H])=C([H])C([H])=C([H])C=1[H])/C([H])([H])C([H])([H])[H]
|
| InChi Key |
NKANXQFJJICGDU-QPLCGJKRSA-N
|
| InChi Code |
InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25-
|
| 化学名 |
2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine
|
| 别名 |
trans-Tamoxifen; Crisafeno; Diemon; Tamoxifene; NSC-180973, Citofen; Istubol; ICI 46474; Nolvadex; ICI-46474; ICI46474; NSC 180973; tamoxifen; tamoxifeni citras; Novaldex
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (13.46 mM) in 30% PEG400 0.5% Tween80 + 5% Propanediol 64.5%H2O (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
配方 2 中的溶解度: 2.5 mg/mL (6.73 mM) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清乙醇储备液加入到 400 μL PEG300 中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.73 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (5.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清的DMSO储备液加入400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 2.08 mg/mL (5.60 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.08 mg/mL (5.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 7 中的溶解度: ≥ 0.83 mg/mL (2.23 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 8 中的溶解度: 0.83 mg/mL (2.23 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 9 中的溶解度: (饱和度未知) in Corn oil:40 mg/mL or 107.67 mM (这些助溶剂从左到右依次添加,逐一添加),*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 10 中的溶解度: 40 mg/mL (107.67 mM) in Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6917 mL | 13.4586 mL | 26.9172 mL | |
| 5 mM | 0.5383 mL | 2.6917 mL | 5.3834 mL | |
| 10 mM | 0.2692 mL | 1.3459 mL | 2.6917 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。