| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Tryptophan hydroxylase
In vitro activity: Telotristat (formerly known as LP-778902) is the active metabolite of LX1606 (Telotristat etiprate) which is an orally bioavailable, small-molecule, tryptophan hydroxylase (TPH) inhibitor with potential an in vivo IC50 of 0.028 μM and with antiserotonergic activity. Telotristat has activity in controlling diarrhea associated with carcinoid syndrome. Telotristat acts by inhibiting the enzyme tryptophan hydoxylase (TPH) and reduces serotonin production both inside and outside the GI tract without affecting brain serotonin levels. Blocking peripheral serotonin synthesis by telotristat reduces severity of both chemical- and infection-induced intestinal inflammation. Kinase Assay: Telotristat (formerly known as LP-778902) is the active metabolite of LX1606 (Telotristat etiprate) which is an orally bioavailable, small-molecule, tryptophan hydroxylase (TPH) inhibitor with potential an in vivo IC50 of 0.028 μM and with antiserotonergic activity. Cell Assay: BON CBA cells are grown in equal volume of DMEM and F12K with 5% bovine serum for 3-4 hours (20 K cell/well) and telotristat is added at a concentration range of 0.07 to 50 μM. The cells are incubated at 37°C overnight. 50 μM of the culture supernatant is then taken for 5HTP measurement. The supernatant is mixed with equal volume of 1M TCA, then filtered through glass fiber. The filtrate is loaded on reverse phase HPLC for 5HTP concentration measurement. The cell viability is measured by treating the remaining cells with Celltiter-Glo Luminescent Cell Viability Assay. |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Telotristat(以前称为 LP-778902)是 LX1606(Telotristat etiprate)的活性代谢物,LX1606 是一种口服生物可利用的小分子色氨酸羟化酶 (TPH) 抑制剂,潜在体内 IC50 为 0.028 μM,并且具有抗血清素活性。 Telotristat 具有控制类癌综合征相关腹泻的活性。 Telotristat 通过抑制色氨酸羟化酶 (TPH) 发挥作用,减少胃肠道内外血清素的产生,而不影响大脑血清素水平。用 telotristat 阻断外周血清素合成可减轻化学和感染引起的肠道炎症的严重程度。激酶测定:Telotristat(以前称为 LP-778902)是 LX1606(Telotristat etiprate)的活性代谢物,LX1606 是一种口服生物可利用的小分子色氨酸羟化酶 (TPH) 抑制剂,体内 IC50 为 0.028 μM,具有抗血清素能作用活动。细胞测定:BON CBA 细胞在等体积的含 5% 牛血清的 DMEM 和 F12K 中生长 3-4 小时(20 K 细胞/孔),并添加浓度范围为 0.07 至 50 μM 的 telotristat。将细胞在 37°C 下孵育过夜。然后取 50 μM 培养上清液用于 5HTP 测量。将上清液与等体积的1M TCA混合,然后通过玻璃纤维过滤。将滤液加载到反相 HPLC 上进行 5HTP 浓度测量。通过用 Celltiter-Glo 发光细胞活力测定处理剩余的细胞来测量细胞活力。
|
| 体内研究 (In Vivo) |
当给予telotristat eth(15、50、150、300mg/kg,po,qd)时,小鼠的外周血而不是大脑中的血清素含量较低。在炎症性肠病大鼠模型中,Telotristat ethyl(200 mg/kg,口服,每日一次)可显着防止 TNBS 诱导的血液中性粒细胞计数升高。组织病理学评估显示,小鼠 IBD 模型受到 telotristat ethyl(200 mg/kg po,qd)的保护[1]。在空肠中,telotristat ethyl (15、50、150 和 300 mg/kg) 消耗 5-HT,但在大脑中则不然。然而,小鼠的组成性胃肠动力和肠神经元血清素(5-HT)的消耗都不是由telotristat ethyl(200 mg/kg,口服)引起的。由三硝基苯磺酸 (TNBS) 引起的结肠炎的严重程度可通过 telotristat ethyl (200 mg/kg) 减轻[2]。
在小鼠中每日口服一次 LX1606(200 mg/kg),持续 6 天,与载体对照组相比,全血中的血清素(5-HT)浓度显著降低 (p<10⁻⁶)。 在 TNBS 诱导的炎症性肠病小鼠模型中,在 TNBS 攻击前 6 天开始并持续进行口服 LX1606(200 mg/kg,每日一次)预处理和治疗,与载体组相比,显著减轻了体重下降 (p<0.03)。 LX1606 治疗显著降低了组织病理学评分 (p=0.04),并减少了病理评分 ≥10 的动物百分比。 它还阻止了载体组在 TNBS 攻击后观察到的血液中性粒细胞计数的增加。 对 LX1606 治疗小鼠远端结肠组织的定量 PCR 分析显示,TNBS 攻击后促炎细胞因子 IL-1β、IL-6 和 IL-17α 的表达有降低趋势。 用 LX1606(200 mg/kg)治疗以剂量依赖性方式降低了小肠中的血清素含量,但不影响大脑中的血清素水平。 [1] |
| 动物实验 |
Dissolved in 15% cyclodextrin or 0.25% methylcellulose; 300 mg/kg; p.o.Male C57BL/6 mice and male C57 albino mice.
In normal mice, telotristat etiprate (administered once daily for 4 days at doses of 15–300 mg/kg/day) was found to reduce serotonin levels throughout the gastrointestinal tract. These reductions occurred in a dose dependent fashion with maximal effects observed with doses of telotristat etiprate ≥150 mg/kg. No significant change in brain serotonin or 5-hydroxyindoleacetic acid (5-HIAA, a serotonin metabolite) was observed. Similar findings were seen in Sprague-Dawley rats. Gastrointestinal motility studies were conducted in rats using the charcoal meal test. There was a significant dose-related delay in both gastrointestinal transit and gastric emptying, associated with a reduction in blood serotonin levels and proximal colon serotonin. A quantitative whole-body autoradiography study was conducted to assess the absorption, distribution and excretion of radioactivity in rats following a single oral dose of telotristat etiprate labeled with carbon 14. Rats were administered either 30 mg/kg or 100 mg/kg of the compound. The distribution of radioactivity was limited to tissues of the hepatic and renal system and the contents of the GI tract. There was no measurable radioactivity in the brain at any dose tested.
C57Bl/6brd x 129SvEv F1 hybrid mice were used in all experiments. For the TNBS-IBD model, animals were challenged via intra-rectal administration with 2% TNBS solution. Naïve control mice were left untreated. LX1606 and the reference drug sulfasalazine were formulated in 0.25% methylcellulose and administered to mice once daily via oral gavage. Dosing began 6 days before TNBS challenge and continued throughout the challenge period. In the efficacy study, mice (n=10 per group initially) were treated with vehicle, LX1606 (200 mg/kg, po, qd), or sulfasalazine (100 mg/kg, po, qd). Body weight was monitored. For serotonin measurement, separate groups of mice (n=5 per group) were treated with indicated doses of LX1606 for 6 days before analysis. Blood was collected for neutrophil counts via retro-orbital bleeding into EDTA tubes, and complete cell counts were measured. At the endpoint, proximal and distal colon and cecum were collected, fixed in formalin, sectioned, and stained with hematoxylin and eosin for histopathological scoring using a modified scoring system. [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After a single oral dose of telotristat ethyl to healthy subjects, telotristat ethyl was absorbed and metabolized to its active metabolite, telotristat. Peak plasma concentrations of telotristat ethyl were achieved within 0.5 to 2 hours, and those of telotristat within 1 to 3 hours. Plasma concentrations thereafter declined in a biphasic manner. Following administration of a single 500 mg dose of telotristat ethyl (twice the recommended dosage) under fasted conditions in healthy subjects, the mean Cmax and AUC0-inf were 4.4 ng/mL and 6.23 ng•hr/mL, respectively for telotristat ethyl. The mean Cmax and AUC0-inf were 610 ng/mL and 2320 ng•hr/mL, respectively for telotristat. Peak plasma concentrations and AUC of telotristat ethyl and telotristat appeared to be dose proportional following administration of a single dose of telotristat ethyl in the range of 100 mg (0.4 times the lowest recommended dose to 1000 mg [4 times the highest recommended dose]) under fasted conditions. Following multiple-dose administration of telotristat ethyl 500 mg three times daily, there was negligible accumulation at steady state for both telotristat ethyl and telotristat. In patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea treated with SSA therapy, the median Tmax for telotristat ethyl and telotristat was approximately 1 and 2 hours, respectively. Following administration of 500 mg telotristat ethyl three times daily, with meals in patients, the mean Cmax and AUC0-6hr were approximately 7 ng/mL and 22 ng•hr/mL, respectively, for telotristat ethyl. The mean Cmax and AUC0-6hr were approximately 900 ng/mL and 3000 ng•hr/mL, respectively for telotristat. The pharmacokinetic parameters for both telotristat ethyl and telotristat were highly variable with about 55% coefficient of variation. Following a single 500 mg oral dose of 14C-telotristat ethyl, 93.2% of the dose was recovered over 240 hours: 92.8% was recovered in the feces, with less than 0.4% being recovered in the urine. The estimated apparent total volume of distribution for the active metabolite from the Population PK model of 428.1 L in a typical healthy fasted subject and 348.7 L in patients with carcinoid syndrome. The apparent total clearance at steady state (CL/Fss) following oral dosing with telotristat ethyl 500 mg three times daily for 14 days (twice the recommended dosage) in healthy subjects was 2.7 and 152 L/hr for telotristat ethyl and telotristat, respectively. Metabolism / Metabolites After oral administration, telotristat ethyl undergoes hydrolysis via carboxylesterases to telotristat, its active metabolite. Telotristat is further metabolized. Among the metabolites of telotristat, the systemic exposure to an acid metabolite of oxidative deaminated decarboxylated telotristat was about 35% of that of telotristat. In vitro data suggest that telotristat ethyl and telotristat are not substrates for CYP enzymes. Biological Half-Life Following a single 500 mg oral dose of telotristat ethyl in healthy subjects, the apparent half-life was approximately 0.6 hours for telotristat ethyl and 5 hours for telotristat. Oral administration of Telotristat Etiprate (LX1606) significantly reduced 5-HT levels in the gut and blood but not in the brain, indicating that the compound is absorbed and acts peripherally but does not cross the blood-brain barrier. The manuscript states that peripheral TPH inhibitors like Telotristat Etiprate (LX1606) do not cross the blood-myenteric plexus barrier, as evidenced by the maintenance of enteric neuronal 5-HT immunoreactivity. Specific pharmacokinetic parameters (e.g., half-life, oral bioavailability, Cmax, AUC) for Telotristat Etiprate (LX1606) are not provided in this manuscript.[2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Both telotristat ethyl and telotristat are greater than 99% bound to human plasma proteins. In the TNBS-IBD study starting with 10 mice per group, 4 mice died by the end of the assay in the vehicle group, while no deaths occurred in the LX1606 or sulfasalazine treatment groups. The cause of death in the vehicle group was likely related to severe IBD. [1] |
| 参考文献 | |
| 其他信息 |
Telotristat ethyl is a prodrug of telotristat that was approved by the FDA in March 2017 as Xermelo. It was previously referred to as telotristat etiprate, the hippurate salt form; however, the FDA recommends the use of the name of the neutral form rather than that of the salt. Currently, telotristat ethyl is used to treat carcinoid syndrome diarrhea from neuroendocrine tumors that are inadequately controlled by short-acting somatostatin analog (SSA) treatment. Neuroendocrine cells are cells that secrete regulatory peptides and biogenic amines in response to chemical, neural, or other types of stimuli. Neuroendocrine tumors (NET) arising from these cells can therefore secrete chemical mediators into the bloodstream to cause side effects in distant sites, a phenomenon called carcinoid syndrome. The most common peptides and amines secreted by NET are histamines, tachykinins, kallikrein, and serotonin. Overexposure to serotonin can cause severe diarrhea, one of the main clinical symptoms of carcinoid syndrome. Serotonin is metabolized in the urinary metabolite 5-hydroxy indole acetic acid (u5-HIAA), and high levels of u5-HIAA is associated with poor survival outcome in patients with NET. The first line treatment of carcinoid syndrome diarrhea is SSA, but symptoms still reoccur over the course of the disease.
Telotristat Ethyl is the ethyl ester form of telotristat, a tryptophan hydroxylase (TPH) inhibitor, with potential anti-serotonergic activity. Upon administration, telotristat binds to and inhibits the activity of TPH. This may result in a reduction in peripheral serotonin (5-HT) production and improvement of serotonin-mediated gastrointestinal effects such as severe diarrhea. TPH, the rate-limiting enzyme in serotonin biosynthesis, is overexpressed in carcinoid tumor cells. See also: Telotristat (has active moiety). Drug Indication Xermelo is indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy. Xermelo is indicated for the treatment of carcinoid syndrome diarrhoea in combination with somatostatin analogue (SSA) therapy in adults inadequately controlled by SSA therapy. Mechanism of Action Telotristat, the active metabolite of telotristat ethyl, is an inhibitor of tryptophan hydroxylase, which mediates the rate-limiting step in serotonin biosynthesis. The in vitro inhibitory potency of telotristat towards tryptophan hydroxylase is 29 times higher than that of telotristat ethyl. Serotonin plays a role in mediating secretion, motility, inflammation, and sensation of the gastrointestinal tract, and is over-produced in patients with carcinoid syndrome. Through inhibition of tryptophan hydroxylase, telotristat and telotristat ethyl reduce the production of peripheral serotonin, and the frequency of carcinoid syndrome diarrhea. Pharmacodynamics In normal mice, telotristat etiprate (administered once daily for 4 days at doses of 15–300 mg/kg/day) was found to reduce serotonin levels throughout the gastrointestinal tract. These reductions occurred in a dose dependent fashion with maximal effects observed with doses of telotristat etiprate ≥150 mg/kg. No significant change in brain serotonin or 5-hydroxyindoleacetic acid (5-HIAA, a serotonin metabolite) was observed. Similar findings were seen in Sprague-Dawley rats. Gastrointestinal motility studies were conducted in rats using the charcoal meal test. There was a significant dose-related delay in both gastrointestinal transit and gastric emptying, associated with a reduction in blood serotonin levels and proximal colon serotonin. A quantitative whole-body autoradiography study was conducted to assess the absorption, distribution and excretion of radioactivity in rats following a single oral dose of telotristat etiprate labeled with carbon 14. Rats were administered either 30 mg/kg or 100 mg/kg of the compound. The distribution of radioactivity was limited to tissues of the hepatic and renal system and the contents of the GI tract. There was no measurable radioactivity in the brain at any dose tested. LX1606 (also known as LX1032 and later as Telotristat ethyl) is a novel, orally delivered inhibitor of tryptophan hydroxylase developed to reduce peripheral serotonin production without affecting central nervous system levels. It is proposed as a potential new therapeutic approach for serotonin-mediated symptoms in conditions like inflammatory bowel disease. The mechanism involves inhibiting TPH1, thereby reducing the synthesis of serotonin from tryptophan in enterochromaffin cells of the gut. [1] |
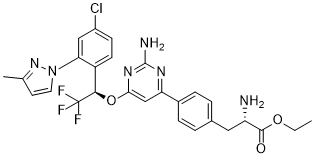
| 分子式 |
C27H26CLF3N6O3
|
|---|---|
| 分子量 |
574.99
|
| 精确质量 |
574.17
|
| 元素分析 |
C, 56.40; H, 4.56; Cl, 6.17; F, 9.91; N, 14.62; O, 8.35
|
| CAS号 |
1033805-22-9
|
| 相关CAS号 |
Telotristat etiprate;1137608-69-5;Telotristat;1033805-28-5; 1033805-22-9 (ethyl); 1374745-52-4 (besilate)
|
| PubChem CID |
25181577
|
| 外观&性状 |
Typically exists as Light yellow to yellow solids at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
704.7±70.0 °C at 760 mmHg
|
| 闪点 |
380.0±35.7 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.621
|
| LogP |
5.47
|
| tPSA |
131.17
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
821
|
| 定义原子立体中心数目 |
2
|
| SMILES |
N[C@@H](CC1=CC=C(C2=NC(N)=NC(O[C@H](C3=CC=C(Cl)C=C3N4N=C(C)C=C4)C(F)(F)F)=C2)C=C1)C(OCC)=O
|
| InChi Key |
MDSQOJYHHZBZKA-GBXCKJPGSA-N
|
| InChi Code |
InChI=1S/C27H26ClF3N6O3/c1-3-39-25(38)20(32)12-16-4-6-17(7-5-16)21-14-23(35-26(33)34-21)40-24(27(29,30)31)19-9-8-18(28)13-22(19)37-11-10-15(2)36-37/h4-11,13-14,20,24H,3,12,32H2,1-2H3,(H2,33,34,35)/t20-,24+/m0/s1
|
| 化学名 |
ethyl (2S)-2-amino-3-[4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl]phenyl]propanoate
|
| 别名 |
LX 1032; LX 1606; LX-1032; LX1606; LX1032; LX-1606; LX1606; LX 1606; Xermelo; Telotristat ethyl [USAN]; trade name: Xermelo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.35 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7392 mL | 8.6958 mL | 17.3916 mL | |
| 5 mM | 0.3478 mL | 1.7392 mL | 3.4783 mL | |
| 10 mM | 0.1739 mL | 0.8696 mL | 1.7392 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。