| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Tryptophan hydroxylase
Telotristat Etiprate (LX 1606 Hippurate) is a selective inhibitor of tryptophan hydroxylase 1 (TPH1), the rate-limiting enzyme for mucosal serotonin (5-HT) synthesis. It exhibits an IC50 of 1.2 nM against recombinant human TPH1 and >1000 nM against TPH2 (neuronal isoform), showing high isoform selectivity [1] - Telotristat Etiprate (LX 1606 Hippurate) targets TPH1 in intestinal epithelial cells and enterochromaffin cells, with no significant binding to TPH2 or other monoamine synthetic enzymes (e.g., tyrosine hydroxylase) at concentrations up to 10 μM [2] |
|---|---|
| 体外研究 (In Vitro) |
Telotristat(以前称为 LP-778902)是 LX1606(Telotristat etiprate)的活性代谢物,LX1606 是一种口服生物可利用的小分子色氨酸羟化酶 (TPH) 抑制剂,潜在体内 IC50 为 0.028 μM,并且具有抗血清素活性。 Telotristat 具有控制类癌综合征相关腹泻的活性。 Telotristat 通过抑制色氨酸羟化酶 (TPH) 发挥作用,减少胃肠道内外血清素的产生,而不影响大脑血清素水平。用 telotristat 阻断外周血清素合成可减轻化学和感染引起的肠道炎症的严重程度。激酶测定:Telotristat(以前称为 LP-778902)是 LX1606(Telotristat etiprate)的活性代谢物,LX1606 是一种口服生物可利用的小分子色氨酸羟化酶 (TPH) 抑制剂,体内 IC50 为 0.028 μM,具有抗血清素能作用活动。细胞测定:BON CBA 细胞在等体积的含 5% 牛血清的 DMEM 和 F12K 中生长 3-4 小时(20 K 细胞/孔),并添加浓度范围为 0.07 至 50 μM 的 telotristat。将细胞在 37°C 下孵育过夜。然后取 50 μM 培养上清液用于 5HTP 测量。将上清液与等体积的1M TCA混合,然后通过玻璃纤维过滤。将滤液加载到反相 HPLC 上进行 5HTP 浓度测量。通过用 Celltiter-Glo 发光细胞活力测定处理剩余的细胞来测量细胞活力。
重组人TPH1活性实验:特利曲坦依普拉酯(0.1-100 nM)呈剂量依赖性抑制TPH1介导的L-色氨酸向5-羟基色氨酸(5-HTP)的转化。在10 nM浓度下,其对TPH1活性的抑制率较溶媒对照组达82%,即使在1 μM浓度下也未检测到对TPH2的抑制作用[1] - Caco-2肠上皮细胞实验:特利曲坦依普拉酯(1-30 nM)处理细胞24小时,可使基础5-羟色胺分泌减少45%-70%(HPLC检测)。当用脂多糖(LPS,1 μg/mL)刺激细胞诱导炎症激活时,该药物(10 nM)可进一步使LPS诱导的5-羟色胺释放减少58%,并使IL-6 mRNA表达降低42%(RT-PCR分析)[1] - 原代小鼠肠道黏膜细胞实验:特利曲坦依普拉酯(3-100 nM)呈剂量依赖性降低黏膜5-羟色胺水平(30 nM时最大降低65%,ELISA检测)。相比之下,TPH2选择性抑制剂(100 nM)对黏膜5-羟色胺无影响,证实TPH1是该药物的作用靶点。该药物对原代肠神经节神经元(表达TPH2)中的5-羟色胺水平也无影响[2] |
| 体内研究 (In Vivo) |
telotristat ethy (15, 50, 150, 300 mg/kg, po, qd) 可降低外周血清素水平,但小鼠大脑中的血清素水平不降低。 Telotristat ethy(200 mg/kg,口服,每日一次)可显着保护小鼠免受炎症性肠病的侵害,并抑制 TNBS 攻击后血液中性粒细胞计数的上升。 IBD 小鼠模型的组织病理学评估证实,telotristat ethy(200 mg/kg,口服,每日一次)可以保护模型[1]。在空肠中,telotristat ethyl (15、50、150 和 300 mg/kg) 消耗 5-HT,但在大脑中则不然。然而,在用telotristat ethyl(200 mg/kg,口服)治疗的小鼠中,既没有发生组成性胃肠动力,也没有发生肠神经元的血清素(5-HT)消耗。由三硝基苯磺酸 (TNBS) 引起的结肠炎的严重程度可通过 telotristat ethyl (200 mg/kg) 减轻 [2]。
DSS诱导的C57BL/6小鼠溃疡性结肠炎模型(饮水中含3% DSS,持续7天):口服给予特利曲坦依普拉酯(10、30、100 mg/kg/天),持续7天,呈剂量依赖性缓解疾病严重程度。在100 mg/kg剂量下,疾病活动指数(DAI,综合体重下降、腹泻和出血评分)从溶媒对照组的4.0降至1.5;结肠长度(炎症诱导缩短的标志物)从6.2 cm增加至8.1 cm(正常约9.0 cm)。组织病理学分析显示,结肠黏膜糜烂面积(从组织面积的85%降至32%)和炎症细胞浸润(中性粒细胞计数减少50%,髓过氧化物酶[MPO]染色检测)均显著降低。结肠组织5-羟色胺水平较对照组降低48%[1] - TNBS诱导的大鼠结肠炎模型(100 mg/kg TNBS灌肠):腹腔注射特利曲坦依普拉酯(30 mg/kg/天),持续5天,可使结肠MPO活性(中性粒细胞浸润指标)从溶媒对照组的21.3 U/mg蛋白降至8.7 U/mg蛋白。血清TNF-α和IL-1β浓度分别降低42%和38%。与非选择性TPH抑制剂不同,特利曲坦依普拉酯不改变脑干(TPH2表达区域)的5-羟色胺水平,也不影响肠道动力(荧光标志物转运时间检测),证实无中枢或神经元脱靶效应[2] |
| 酶活实验 |
重组人TPH1活性实验:200 μL反应体系包含50 mM Tris-HCl(pH 7.5)、0.1 mM FeSO4、0.5 mM(6R)-L-赤藓糖-5,6,7,8-四氢生物蝶呤(BH4,辅酶)、100 μM L-色氨酸(底物)、50 ng重组人TPH1蛋白,以及浓度为0.1、0.3、1、3、10、30或100 nM的特利曲坦依普拉酯(溶媒对照:0.1% DMSO)。混合物在37°C孵育30分钟,加入50 μL 10%三氯乙酸(TCA)终止反应。离心(4°C,10,000×g,10分钟)去除沉淀蛋白,上清液通过高效液相色谱(HPLC)荧光检测(激发波长:280 nm;发射波长:340 nm)定量5-HTP生成量。TPH1活性以每毫克TPH1每小时生成的5-HTP纳摩尔数计算,抑制率以溶媒对照组为基准确定。通过非线性回归(四参数逻辑模型)计算IC50[1]
- TPH2选择性实验:实验方案与TPH1实验一致,差异在于使用50 ng重组人TPH2蛋白,且特利曲坦依普拉酯浓度范围为100 nM至10 μM。结果显示,即使在10 μM浓度下,TPH2活性抑制率也<5%,证实亚型选择性[1] |
| 细胞实验 |
Caco-2细胞5-羟色胺分泌实验:将Caco-2细胞(20-35代)以5×104细胞/孔接种于24孔板,在含10%胎牛血清、1%非必需氨基酸和抗生素的DMEM培养基中(37°C,5% CO2)培养至融合(7天)。更换为含特利曲坦依普拉酯(1、3、10、30 nM)或溶媒(0.1% DMSO)的无血清DMEM,孵育24小时。LPS刺激组在孵育最后6小时加入1 μg/mL LPS。收集培养上清液,通过反相HPLC(C18柱,流动相:0.1%三氟乙酸水溶液/乙腈=90:10,流速:1 mL/min)电化学检测5-羟色胺浓度。通过MTT实验评估细胞活力,确认实验浓度下无细胞毒性[1]
- 原代小鼠肠道黏膜细胞分离与5-羟色胺检测:从C57BL/6小鼠空肠刮取肠道黏膜,用胶原酶(0.1% w/v)在37°C消化30分钟,过滤获得单细胞悬液。细胞以1×105细胞/孔接种于96孔板,用特利曲坦依普拉酯(3、10、30、100 nM)或TPH2抑制剂(100 nM)处理18小时。用0.1 M高氯酸裂解细胞,离心(4°C,12,000×g,15分钟),上清液通过竞争性ELISA试剂盒定量5-羟色胺水平,结果以总蛋白浓度(BCA法)归一化[2] |
| 动物实验 |
Dissolved in 15% cyclodextrin or 0.25% methylcellulose; 300 mg/kg; p.o.
Male C57BL/6 mice and male C57 albino mice. Animal information: C57Bl/6brd x 129SvEv F1 hybrid mice were used in all experiments. The studies were carried out with protocols approved by the Institutional Animal Care and Use Committee of Lexicon Pharmaceuticals, Inc.[1] 5-HT measurement: Blood was mixed in buffer containing 56 mM sodium ascorbate and 600 mM trichloroacetic acid, and jejunum tissues were homogenized in a buffer containing 300 mM trichloroacetic acid, 100 mM sodium acetate, pH 3.5, 0.01 mM EDTA, and 20 mM sodium bisulfate. The resulting cell lysates were centrifuged and the supernatants analyzed for 5-HT content using reverse phase HPLC with a C18 column and an in-line fluorescence detector. [1] TNBS-IBD model: Animals were challenged via intra-rectal administration with 2% TNBS or left untreated as naïve controls. LX1606 and sulfasalazine were formulated in 0.25% methylcellulose and given to mice once daily via oral gavage starting 6 days before TNBS challenge and continuing during challenge. [1] Blood neutrophil count: Blood was collected in EDTA by retro-orbital bleeding and complete cell counts were measured on a Veterinary cell counter. [1] Histological analysis: Proximal and distal colon and cecum were collected, fixed in formalin, and sections were stained with hematoxylin and eosin. The sections were scored using a modified TJL system. [1] Quantitative polymerase chain reaction (qPCR) analysis of cytokine expression: Total RNA was extracted from distal colon. Interested genes were analyzed by standard qPCR methods[1] Two peripheral TPH inhibitors, LP-920540 and telotristat etiprate (LX1032; LX1606) were given orally to mice. Effects were measured on 5-HT levels in the gut, blood and brain, 5-HT immunoreactivity in the ENS, gastrointestinal motility and severity of trinitrobenzene sulfonic acid (TNBS)-induced colitis. Quantitation of clinical scores, histological damage and intestinal expression of inflammation-associated cytokines and chemokines with focused microarrays and real-time reverse transcriptase PCR were employed to evaluate the severity of intestinal inflammation.[2] Results: LP-920540 and LX1032 reduced 5-HT significantly in the gut and blood but not in the brain. Neither LP-920540 nor LX1032 decreased 5-HT immunoreactive neurons or fibres in the myenteric plexus and neither altered total gastrointestinal transit time, colonic motility or gastric emptying in mice. In contrast, oral LP-920540 and LX1032 reduced the severity of TNBS-induced colitis; the expression of 24% of 84 genes encoding inflammation-related cytokines and chemokines was lowered at least fourfold and the reduced expression of 17% was statistically significant.[2] DSS-Induced Murine Ulcerative Colitis Model: Female C57BL/6 mice (6-8 weeks old, 18-22 g) were housed under SPF conditions (22±2°C, 12-hour light/dark cycle, free access to food/water). Mice were randomly divided into 4 groups (n=8/group): normal control (no DSS, vehicle), DSS control (3% DSS in drinking water, vehicle), and DSS + Telotristat Etiprate (10, 30, 100 mg/kg/day). Telotristat Etiprate was dissolved in 0.5% carboxymethylcellulose sodium (CMC-Na) and administered via oral gavage once daily for 7 days, starting concurrently with DSS exposure. On day 8, mice were euthanized; colons were excised, measured for length, and divided into segments: one segment was fixed in 4% paraformaldehyde for histopathology (H&E staining), another was homogenized for MPO activity assay (using a colorimetric kit) and serotonin quantification (HPLC), and a third was used for RNA extraction (RT-PCR for IL-6, TNF-α) [1] - TNBS-Induced Rat Colitis Model: Male Sprague-Dawley rats (200-250 g) were anesthetized with isoflurane (2% induction, 1% maintenance). A TNBS solution (100 mg/kg in 50% ethanol) was administered via intrarectal enema (5 cm from anus) to induce colitis. Rats were randomized to 3 groups (n=6/group): sham control (saline enema, vehicle), TNBS control (TNBS enema, vehicle), and TNBS + Telotristat Etiprate (30 mg/kg/day). Telotristat Etiprate was dissolved in saline and injected intraperitoneally once daily for 5 days, starting 24 hours after TNBS administration. On day 6, rats were euthanized: blood was collected for serum cytokine analysis (ELISA), colons were harvested for MPO activity and serotonin assays, and brainstems were dissected to measure serotonin levels (HPLC). Intestinal motility was assessed 1 day before euthanasia by gavage of a fluorescent dextran (100 mg/kg) and measuring transit time to fecal excretion [2] |
| 药代性质 (ADME/PK) |
In mice after oral administration of Telotristat Etiprate (100 mg/kg): Plasma drug concentration reached a peak (Cmax) of 256 ng/mL at 1.5 hours (Tmax), with an elimination half-life (t1/2) of 4.2 hours. Colonic mucosal drug concentration was 1210 ng/g tissue at Tmax, 4.7-fold higher than plasma concentration, indicating preferential distribution to the target tissue (intestine). Oral bioavailability was calculated as 35% (compared to intravenous administration of 10 mg/kg) [1]
- In rats after intraperitoneal injection of Telotristat Etiprate (30 mg/kg): Plasma Cmax was 189 ng/mL at 0.8 hours, t1/2 was 3.8 hours. Minimal drug penetration into the central nervous system was observed: brainstem drug concentration was <10 ng/g tissue at all time points, consistent with TPH2 selectivity [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute toxicity in mice: Single oral doses of Telotristat Etiprate (100, 300, 1000 mg/kg) caused no mortality or abnormal behavior over 7 days. At 1000 mg/kg, transient reduced food intake was observed on day 1 but resolved by day 2; no changes in serum ALT, AST, BUN, or creatinine were detected compared to controls [1]
- Subacute toxicity in rats: Daily intraperitoneal injection of Telotristat Etiprate (30, 100 mg/kg) for 14 days showed no significant differences in body weight, organ weights (liver, kidney, colon), or histopathological findings in major organs. Colonic epithelial integrity (measured via transepithelial electrical resistance in ex vivo colon segments) was preserved, indicating no intestinal toxicity [2] |
| 参考文献 | |
| 其他信息 |
Telotristat Etiprate is an orally bioavailable, small-molecule, tryptophan hydroxylase (TPH) inhibitor prodrug, with potential antiserotonergic activity. Upon administration, telotristat etiprate is converted to its active moiety, telotristat (LP-778902), which binds to and blocks the activity of TPH. This may result in a reduction in peripheral serotonin (5-HT) production and improvement of serotonin-mediated gastrointestinal effects such as severe diarrhea. TPH, the rate-limiting enzyme in serotonin biosynthesis, is overexpressed in carcinoid tumor cells.
See also: Telotristat (has active moiety). Drug Indication Xermelo is indicated for the treatment of carcinoid syndrome diarrhoea in combination with somatostatin analogue (SSA) therapy in adults inadequately controlled by SSA therapy. Treatment of carcinoid syndrome Telotristat Etiprate (LX 1606 Hippurate) is a first-in-class selective TPH1 inhibitor designed to target mucosal serotonin overproduction, a key driver of intestinal inflammation in inflammatory bowel disease (IBD). Its high selectivity for TPH1 (vs. TPH2) avoids disrupting neuronal serotonin signaling (critical for mood, sleep, and intestinal motility), reducing the risk of central or gastrointestinal side effects associated with non-selective TPH inhibitors [1,2] - In preclinical IBD models [1], Telotristat Etiprate demonstrated both anti-inflammatory and tissue-protective effects, reducing mucosal damage and inflammatory cytokine release independently of conventional immune suppression, suggesting potential utility in patients with refractory IBD or those intolerant to anti-TNF therapies [1] - The drug’s preferential distribution to intestinal mucosa [1] and lack of central penetration [2] support its use as a local intestinal therapeutic, minimizing systemic exposure and off-target effects. Beyond IBD, preclinical data suggest potential applications in other serotonin-mediated gastrointestinal disorders, such as carcinoid syndrome-related diarrhea (a condition characterized by excessive mucosal serotonin secretion) [1] - In TNBS-induced colitis [2], Telotristat Etiprate’s ability to reduce inflammation without impairing intestinal motility addresses a major limitation of some IBD therapies (e.g., opioids, which cause constipation), highlighting its favorable safety profile for long-term use [2] |
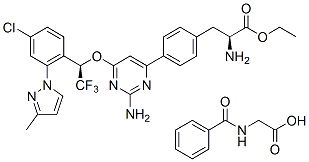
| 分子式 |
C27H26CLF3N6O3.C9H9NO3
|
|
|---|---|---|
| 分子量 |
754.15
|
|
| 精确质量 |
753.228
|
|
| 元素分析 |
C, 57.33; H, 4.68; Cl, 4.70; F, 7.56; N, 13.00; O, 12.73
|
|
| CAS号 |
1137608-69-5
|
|
| 相关CAS号 |
Telotristat ethyl;1033805-22-9;Telotristat;1033805-28-5; 1137608-69-5 (etiprate) ; 1374745-52-4 (besilate)
|
|
| PubChem CID |
25253377
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 熔点 |
145 °C
|
|
| LogP |
7.162
|
|
| tPSA |
197.57
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
14
|
|
| 可旋转键数目(RBC) |
13
|
|
| 重原子数目 |
53
|
|
| 分子复杂度/Complexity |
1020
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
O=C([C@H](CC1=CC=C(C=C1)C2=CC(O[C@H](C3=C(C=C(C=C3)Cl)N4C=CC(C)=N4)C(F)(F)F)=NC(N)=N2)N)OCC.O=C(C5=CC=CC=C5)NCC(O)=O
|
|
| InChi Key |
XSFPZBUIBYMVEA-CELUQASASA-N
|
|
| InChi Code |
InChI=1S/C27H26ClF3N6O3.C9H9NO3/c1-3-39-25(38)20(32)12-16-4-6-17(7-5-16)21-14-23(35-26(33)34-21)40-24(27(29,30)31)19-9-8-18(28)13-22(19)37-11-10-15(2)36-37;11-8(12)6-10-9(13)7-4-2-1-3-5-7/h4-11,13-14,20,24H,3,12,32H2,1-2H3,(H2,33,34,35);1-5H,6H2,(H,10,13)(H,11,12)/t20-,24+;/m0./s1
|
|
| 化学名 |
2-benzamidoacetic acid;ethyl (2S)-2-amino-3-[4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl]phenyl]propanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 10 mg/mL (13.26 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 100.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 10 mg/mL (13.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 100.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3260 mL | 6.6300 mL | 13.2600 mL | |
| 5 mM | 0.2652 mL | 1.3260 mL | 2.6520 mL | |
| 10 mM | 0.1326 mL | 0.6630 mL | 1.3260 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03442725 | Completed Has Results | Drug: Telotristat etiprate | Renal Impairment | Ipsen | February 9, 2018 | Phase 1 |
| NCT02026063 | Completed Has Results | Drug: Telotristat etiprate | Carcinoid Syndrome | Lexicon Pharmaceuticals | January 14, 2014 | Phase 3 |
| NCT02063659 | Completed Has Results | Drug: Telotristat etiprate Drug: Placebo |
Carcinoid Syndrome | Lexicon Pharmaceuticals | March 11, 2014 | Phase 3 |
| NCT01104415 | Completed Has Results | Drug: Telotristat etiprate | Carcinoid Syndrome | Lexicon Pharmaceuticals | June 15, 2010 | Phase 2 |
|