| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
在体外,特比萘芬主要作为杀菌剂,对抗多种真菌病害,例如丝状真菌病、二形真菌病和皮肤癣菌病。在角鲨烯环氧化位点,特比萘芬选择性抑制真菌麦角甾醇的形成。中间体角鲨烯通过处理的真菌细胞快速积累[1]。
|
|---|---|
| 体内研究 (In Vivo) |
特比萘芬已被证明在局部应用和口服时对实验性皮肤癣菌病特别有效。在第四次特比萘芬治疗期间,患有真菌的豚鼠的皮肤温度显着下降[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral terbinafine is >70% absorbed but only 40% bioavailable after first pass metabolism, reaching a Cmax of 1µg/mL with a Tmax of 2 hours an an AUC of 4.56µg\*h/mL. Over the course of a week, 1% topical terbinafine's Cmax increases from 949-1049ng/cm2 and the AUC increases from 9694-13,492ng/cm2/h. Terbinafine is approximately 80% eliminated in urine, while the remainder is eliminated in feces. The unmetabolized parent drug is not present in urine. A single 250mg oral dose of terbinafine has a volume of distribution at steady state of 947.5L or 16.6L/kg. A single 250mg oral dose of terbinafine has a clearance of 76L/h or 1.11L/h/kg. Metabolism / Metabolites Terbinafine can be deaminated to 1-naphthaldehyde by CYP2C9, 2B6, 2C8, 1A2, 3A4, and 2C19. 1-naphthaldehyde is then oxidized to 1-naphthoic acid or reduced to 1-naphthalenemethanol. Terbinafine can also be hydroxylated by CYP1A2, 2C9, 2C8, 2B6, and 2C19 to hydroxyterbinafine. Hydroxyterbinafine is then oxidized to carboxyterbinafine or N-demethylated by CYP3A4, 2B6, 1A2, 2C9, 2C8, and 2C19 to desmethylhydroxyterbinafine. Terbinafine can be N-demethylated to desmethylterbinafine. Desmethylterbinafine is then dihydroxylated to a desmethyldihydrodiol or hydroxylated to desmethylhydroxyterbinafine. Finally, terbinafine can be dihydroxylated to a dihydrodiol which is then N-demethylated to a desmethyldihydrodiol. Terbinafine has known human metabolites that include Hydroxyterbinafine, N-Desmethylterbinafine, and 1-Naphtaldehyde. Biological Half-Life Oral terbinafine has an effective half life of approximately 36 hours. However, the terminal half life ranges from 200-400 hours as it distributes into skin and adipose tissue. 1% topical terbinafine's half life increases over the first seven days from approximately 10-40 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Drug induced liver injury due to terbinafine was identified shortly after its introduction into medical use. Oral therapy with terbinafine is associated with elevations in serum aminotransferases in less than 1% of patients and the elevations are generally asymptomatic and resolve without stopping therapy. The estimated probability of developing elevated serum aminotransferase levels requiring stopping treatment is about 0.31% for 2 to 6 weeks' treatment and 0.44% for treatment longer than 8 weeks. Clinically apparent liver injury from terbinafine occurs rarely (1 in 50,000 to 120,000 prescriptions), but many case reports and even case series have been described in the literature. Liver injury usually arises within the first 6 weeks of therapy. The pattern of injury can be either hepatocellular or cholestatic initially, but typically evolves into a cholestatic pattern which can be prolonged (Cases 1 and 2). Some cases may progress to vanishing bile duct syndrome. Signs of hypersensitivity (rash, fever, eosinophilia) are not common and, when present, are generally mild-to-moderate in severity. Autoantibody formation is rare. In addition, cases with severe hepatocellular injury with acute liver failure have been described. These instances are marked by precipitous onset with marked elevations in serum aminotransferase levels and progressive jaundice and hepatic failure. Terbinafine has also been implicated in cases of Stevens-Johnson syndrome, in which case the hepatic injury may be overshadowed by rash and allergic symptoms. Likelihood score: B (highly likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that oral maternal doses of 500 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. Monitor the infant for jaundice or other signs of liver toxicity, especially in younger, exclusively breastfed infants. Some sources recommend avoiding oral terbinafine during nursing. Topical terbinafine has not been studied during breastfeeding. Because only about 1% is absorbed after topical application, it is considered a low risk to the nursing infant. Avoid application to the nipple area and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only water-miscible cream, gel or liquid products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Terbinafine is >99% bound to proteins in plasma, mostly to serum albumin, high and low density lipoproteins, and alpha-1-acid glycoprotein to a lesser extent. |
| 参考文献 | |
| 其他信息 |
Terbinafine is a tertiary amine that is N-methyl-1-naphthalenemethylamine in which the amino hydrogen is replaced by a 3-(tertbutylethynyl)allyl group. An antifungal agent administered orally (generally as the hydrochloride salt) for the treatment of skin and nail infections. It has a role as an EC 1.14.13.132 (squalene monooxygenase) inhibitor, a P450 inhibitor and a sterol biosynthesis inhibitor. It is a tertiary amine, an acetylenic compound, a member of naphthalenes, an enyne and an allylamine antifungal drug. It is a conjugate base of a terbinafine(1+).
Terbinafine hydrochloride (Lamisil) is a synthetic allylamine antifungal. It is highly lipophilic in nature and tends to accumulate in skin, nails, and fatty tissues. Like other allylamines, terbinafine inhibits ergosterol synthesis by inhibiting the fungal squalene monooxygenase (also called squalene epoxidase), an enzyme that is part of the fungal cell wall synthesis pathway. Terbinafine hydrochloride was granted FDA approval on 30 December 1992. Terbinafine is an Allylamine Antifungal. Terbinafine is an orally and topically active allylamine fungicidal agent which is used to treat superficial fungal infections of the skin and nails. Terbinafine has been clearly linked to rare instances of acute liver injury that can be severe and sometimes fatal. Terbinafine is a synthetic allylamine derivative with antifungal activity. Terbinafine exerts its effect through inhibition of squalene epoxidase, thereby blocking the biosynthesis of ergosterol, an important component of fungal cell membranes. As a result, this agent disrupts fungal cell membrane synthesis and inhibits fungal growth. A naphthalene derivative that inhibits fungal SQUALENE EPOXIDASE and is used to treat DERMATOMYCOSES of the skin and nails. See also: Terbinafine Hydrochloride (active moiety of); Betamethasone Acetate; Florfenicol; Terbinafine (component of); Florfenicol; Mometasone furoate; Terbinafine (component of) ... View More ... Drug Indication Terbinafine hydrochloride is indicated to treat fungal skin and nail infections caused by _Trichophyton_ species, _Microsporum canis_, _Epidermophyton floccosum_, and _Tinea_ species. Terbinafine hydrochloride also treats yeast infections of the skin caused by _Candida_ species and _Malassezia furfur_. FDA Label Mechanism of Action Terbinafine inhibits the enzyme squalene monooxygenase (also called squalene epoxidase), preventing the conversion of squalene to 2,3-oxydosqualene, a step in the synthesis of ergosterol. This inhibition leads to decreased ergosterol, which would normally be incorporated into the cell wall, and accumulation of squalene. Generation of a large number of squalene containing vesicles in the cytoplasm may leach other lipids away from, and further weaken, the cell wall. Pharmacodynamics Terbinafine is an allylamine antifungal that inhibits squalene epoxidase (also known as squalene monooxygenase) to prevent the formation of ergosterol and cause an accumulation of squalene, weakening the cell wall of fungal cells. Terbinafine distributes into tissues and has a long terminal elimination half life, so the duration of action is long. Overdose with terbinafine is rare, even above the therapeutic dose, so the therapeutic index is wide. Patients taking oral terbinafine should have liver function tests performed prior to treatment to reduce the risk of liver injury. |
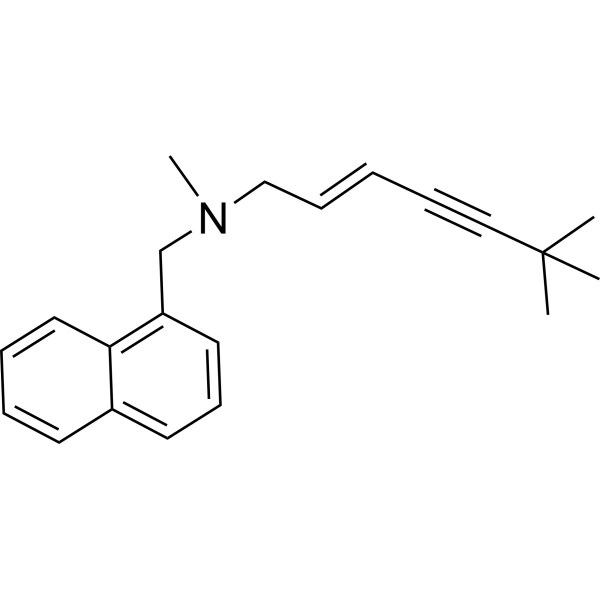
| 分子式 |
C21H25N
|
|---|---|
| 分子量 |
291.4299
|
| 精确质量 |
291.198
|
| CAS号 |
91161-71-6
|
| 相关CAS号 |
Terbinafine hydrochloride;78628-80-5;Terbinafine-d3 hydrochloride;1310012-15-7;Terbinafine-d7;1185240-27-0;Terbinafine lactate;335276-86-3
|
| PubChem CID |
1549008
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
417.9±33.0 °C at 760 mmHg
|
| 闪点 |
183.7±22.3 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.586
|
| LogP |
6.61
|
| tPSA |
3.24
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
428
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)(C)C#C/C=C/CN(C)CC1=CC=CC2=CC=CC=C21
|
| InChi Key |
DOMXUEMWDBAQBQ-WEVVVXLNSA-N
|
| InChi Code |
InChI=1S/C21H25N/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19/h5-7,9-14H,16-17H2,1-4H3/b9-5+
|
| 化学名 |
(E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~343.14 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (8.58 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.5 mg/mL (8.58 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 配方 4 中的溶解度: 16.25 mg/mL (55.76 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4314 mL | 17.1568 mL | 34.3136 mL | |
| 5 mM | 0.6863 mL | 3.4314 mL | 6.8627 mL | |
| 10 mM | 0.3431 mL | 1.7157 mL | 3.4314 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Comparative Study Between Pulse Therapy With Oral Itraconazole Versus Continuous Oral Terbinafine Therapy for Treatment of Onychomycosis
CTID: NCT05578950
Phase: Phase 1 Status: Completed
Date: 2022-10-19