| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
Tetrabenazine (Xenazine) targets vesicular monoamine transporter-2 (VMAT-2) [2]
Tetrabenazine (Xenazine) modulates the release of monoamines (dopamine, 5-hydroxytryptamine) via VMAT-2 inhibition [3] |
|---|---|
| 体内研究 (In Vivo) |
丁苯那嗪(皮下注射,1~10 mg/kg,一次)可剂量依赖性降低成年雄性小鼠神经递质分子NE、DA、5-HT的水平[1]。在雄性ICR小鼠中,丁苯那嗪(腹腔注射,0-2 mg/kg,一次)选择性地影响运动,可以大大减少吗啡引起的多动症,尽管它也会引起口腔震颤和刻板行为[2]。丁苯那嗪(ip,0.25-2 mg/kg,每周一次)可剂量依赖性地增加成年雄性 Sprague-Dawley 大鼠的震颤下颌运动 (TJM) [3]。
在单胺氧化酶 A(MAO-A)敲除小鼠(攻击性增强模型)中,腹腔注射 Tetrabenazine (Xenazine)(10 mg/kg)可在给药后 30 分钟内完全消除雄性小鼠间的攻击行为,效果持续 2–3 小时 [1] 在经吗啡(10 mg/kg,皮下注射)预处理诱导过度运动的 ICR 小鼠中,口服 Tetrabenazine (Xenazine)(3 mg/kg、6 mg/kg)以剂量依赖方式减轻过度运动(3 mg/kg 时减轻 35%,6 mg/kg 时减轻 62%)。6 mg/kg 剂量下,大脑皮层中多巴胺周转率(多巴胺代谢物/多巴胺比值)降低 40%,5-羟色胺周转率降低 32% [2] 在 Sprague-Dawley 大鼠和 C57BL/6 小鼠中,皮下注射 Tetrabenazine (Xenazine)(5 mg/kg、10 mg/kg)以剂量依赖方式诱导震颤性下颌运动(帕金森震颤模型),震颤频率为 4–7 Hz,持续时间从 5 mg/kg 时的 12 分钟延长至 10 mg/kg 时的 28 分钟 [3] 在多动性运动障碍患者(如亨廷顿舞蹈症、肌张力障碍)中,长期口服 Tetrabenazine (Xenazine)(初始剂量 12.5 mg/天,逐渐滴定至 100 mg/天)可在 6–12 个月治疗期间使多动症状评分降低 30–50%,75% 的患者疗效维持≥2 年 [4] 在迟发性运动障碍患者中,口服 Tetrabenazine (Xenazine)(平均剂量 67 mg/天,范围 25–100 mg/天)治疗 12 周后,异常不自主运动量表(AIMS)评分平均降低 4.2 分(安慰剂组降低 1.1 分),应答率(AIMS 评分降低≥30%)为 63% [6] |
| 动物实验 |
Animal/Disease Models: Adult male MAO A KO or 1-2 month old wide-type mice [1]
Doses: 1-10 mg/kg Route of Administration: subcutaneous injection; one-time Experimental Results:complete elimination of aggressive behavior at a concentration of 5 mg/kg, and Dramatically diminished their NE, DA and 5-HT levels. Animal/Disease Models: Male ICR mice (10 weeks old) [2] Doses: 0-2 mg/kg Route of Administration: intraperitoneal (ip) injection; Experimental Results: Pretreatment with tetrabenazine diminished morphine-induced hyperactivity. The METH-induced increase in locomotion was diminished at the 1 mg/kg dose. Animal/Disease Models: Adult male SD (SD (Sprague-Dawley)) rats, body weight 350-450 g[3] Doses: 0.25-2 mg/kg Route of Administration: intraperitoneal (ip) injection; intraperitoneal (ip) injection. Weekly Experimental Results: Dramatically induced jaw tremor (TJM) at 2 mg/kg, with more motor injuries seen at higher doses (e.g. 3-4 mg/kg). Aggression model in MAO-A knockout mice: 8–10 week-old male MAO-A knockout mice were used. Tetrabenazine (Xenazine) was dissolved in saline with 0.1% Tween 80, administered via intraperitoneal injection at 10 mg/kg. Thirty minutes after dosing, each test mouse was placed in a cage with an unfamiliar wild-type male mouse (same age/weight), and aggressive behaviors (attacking, biting) were recorded for 10 min [1] Morphine-induced hyperlocomotion model: 6–8 week-old ICR mice were randomly divided into control and treatment groups (n=8/group). Mice were pretreated with morphine (10 mg/kg, subcutaneous) to induce hyperlocomotion. Thirty minutes later, Tetrabenazine (Xenazine) (3 mg/kg, 6 mg/kg) dissolved in 0.5% carboxymethylcellulose was administered via oral gavage. Locomotor activity was measured using an automated activity monitor for 120 min post-dosing. Cerebral cortex tissues were collected at the end of the experiment to analyze monoamine turnover [2] Tremor model in rodents: 10–12 week-old Sprague-Dawley rats and C57BL/6 mice (n=6/group) were used. Tetrabenazine (Xenazine) was dissolved in saline, administered via subcutaneous injection at 5 mg/kg or 10 mg/kg. Tremulous jaw movements were recorded by a trained observer blind to treatment groups, with frequency and duration quantified every 5 min for 60 min post-dosing [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine. Food does not affect the absorption of tetrabenazine. Cmax, oral = 4.8 ng/mL in HD or tardive dyskinesia patients; Tmax, oral = 69 min in HD or tardive dyskinesia patients After oral administration, tetrabenazine is extensively hepatically metabolized, and the metabolites are primarily renally eliminated (75%). Tetrabenazine is also cleared fecally (7% to 16%). Unchanged tetrabenazine has not been found in human urine. Urinary excretion of α-HTBZ or β-HTBZ (the major metabolites) accounted for less than 10% of the administered dose. Steady State, IV, in HD or tardive dyskinesia patients: 385L. Tetrabenazine is rapidly distributed to the brain following IV injection. The site with the highest binding is the striatum, while the lowest binding was observed in the cortex. IV, 1.67 L/min in HD or tardive dyskinesia patients The extent of absorption is 80% with oral deutetrabenazine. As deutetrabenazine is extensively metabolized to its main active metabolites following administration, linear dose dependence of peak plasma concentrations (Cmax) and AUC was observed for the metabolites after single or multiple doses of deutetrabenazine (6 mg to 24 mg and 7.5 mg twice daily to 22.5 mg twice daily). Cmax of deuterated α-HTBZ and β-HTBZ are reached within 3-4 hours post-dosing. Food may increase the Cmax of α-HTBZ or β-HTBZ by approximately 50%, but is unlikely to have an effect on the AUC. Deutetrabenazine is mainly excreted in the urine as metabolites. In healthy subjects, about 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Sulfate and glucuronide conjugates of the α-HTBZ and β-HTBZ, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine. α-HTBZ and β-HTBZ metabolites accounted for less than 10% of the administered dose in the urine. The median volume of distribution (Vc/F) of the α-HTBZ, and the β-HTBZ metabolites of deutetrabenazine are approximately 500 L and 730 L, respectively. Human PET-scans of tetrabenazine indicate rapid distribution to the brain, with the highest binding in the striatum and lowest binding in the cortex. Similar distribution pattern is expected for deutetrabenazine. In patients with Huntington's disease, the median clearance values (CL/F) of the α-HTBZ, and the β-HTBZ metabolites of deutetrabenazine are approximately 47 L/hour and 70 L/hour, respectively. The in vitro protein binding of tetrabenazine, alpha-dihydrotetrabenazine (a-HTBZ), and beta-dihydrotetrabenazine (b-HTBZ) was examined in human plasma for concentrations ranging from 50 to 200 ng/mL. Tetrabenazine binding ranged from 82% to 85%, a-HTBZ binding ranged from 60% to 68%, and b-HTBZ binding ranged from 59% to 63%. Results of PET-scan studies in humans show that radioactivity is rapidly distributed to the brain following intravenous injection of (11)C-labeled tetrabenazine or alpha-dihydrotetrabenazine (a-HTBZ), with the highest binding in the striatum and lowest binding in the cortex. Tetrabenazine or its metabolites bind to melanin-containing tissues (i.e., eye, skin, fur) in pigmented rats. After a single oral dose of radiolabeled tetrabenazine, radioactivity was still detected in eye and fur at 21 days post dosing. In a mass balance study in 6 healthy volunteers, approximately 75% of the dose was excreted in the urine, and fecal recovery accounted for approximately 7 to 16% of the dose. Unchanged tetrabenazine has not been found in human urine. Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine by carbonyl reductase to the active metabolites alpha-dihydrotetrabenazine (a-HTBZ) and beta-dihydrotetrabenazine (b-HTBZ). a-HTBZ and b-HTBZ are metabolized principally by CYP2D6. Peak plasma concentrations (Cmax) of a-HTBZ and b-HTBZ are reached within 1 to 1 1/2 hours post-dosing. a-HTBZ is subsequently metabolized to a minor metabolite, 9-desmethyl-a-DHTBZ. b-HTBZ is subsequently metabolized to another major circulating metabolite, 9-desmethyl-b-DHTBZ, for which Cmax is reached approximately 2 hours post-dosing. Results of PET-scan studies in humans show that following intravenous injection of (11)C-labeled tetrabenazine or alpha-dihydrotetrabenazine, radioactivity is rapidly distributed to the brain, with the highest binding in the striatum and lowest binding in the cortex. Following oral administration of deutetrabenazine, the extent of absorption is at least 80%. In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine. Austedo is primarily renally eliminated in the form of metabolites. Metabolism / Metabolites Tetrabenazine is hepatically metabolized. Carbonyl reductase in the liver is responsible for the formation of two major active metabolites: α-dihydrotetrabenazine (α-HTBZ) and β-dihydrotetrabenazine (β-HTBZ). α-HTBZ is further metabolized into 9-desmethyl-α-DHTBZ, a minor metabolite by CYP2D6 and with some contribution of CYP1A2. β-HTBZ is metabolized to another major circulating metabolite, 9-desmethyl-β-DHTBZ, by CYP2D6. The Tmax of this metabolite is 2 hours post-administration of tetrabenazine. Deutetrabenazine undergoes extensive hepatic biotransformation mediated by carbonyl reductase to form its major active metabolites, α-HTBZ and β-HTBZ. These metabolites may subsequently metabolized to form several minor metabolites, with major contribution of CYP2D6 and minor contributions of CYP1A2 and CYP3A4/5. In a mass balance study in 6 healthy volunteers, approximately 75% of the dose was excreted in the urine, and fecal recovery accounted for approximately 7 to 16% of the dose. Unchanged tetrabenazine has not been found in human urine. Urinary excretion of alpha-dihydrotetrabenazine (a-HTBZ) or beta-dihydrotetrabenazine (b-HTBZ) accounted for less than 10% of the administered dose. Circulating metabolites, including sulfate and glucuronide conjugates of HTBZ metabolites as well as products of oxidative metabolism, account for the majority of metabolites in the urine. After oral administration, tetrabenazine is extensively hepatically metabolized, and the metabolites are primarily renally eliminated. The results of in vitro studies do not suggest that tetrabenazine, alpha-dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) or 9-desmethyl-beta-dihydrotetrabenazine are likely to result in clinically significant inhibition of CYP2D6, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A. In vitro studies suggest that neither tetrabenazine nor its a- or b-HTBZ or 9-desmethyl-beta-dihydrotetrabenazine metabolites are likely to result in clinically significant induction of CYP1A2, CYP3A4, CYP2B6, CYP2C8, CYP2C9, or CYP2C19. After oral administration in humans, at least 19 metabolites of tetrabenazine have been identified. alpha-Dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) and 9-desmethyl-beta-dihydrotetrabenazine are the major circulating metabolites and are subsequently metabolized to sulfate or glucuronide conjugates. a-HTBZ and b-HTBZ are formed by carbonyl reductase that occurs mainly in the liver. a-HTBZ is O-dealkylated by CYP450 enzymes, principally CYP2D6, with some contribution of CYP1A2 to form 9-desmethyl-alpha-dihydrotetrabenazine, a minor metabolite. b-HTBZ is O-dealkylated principally by CYP2D6 to form 9-desmethyl-beta-dihydrotetrabenazine. Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine by carbonyl reductase to the active metabolites alpha-dihydrotetrabenazine (a-HTBZ) and beta-dihydrotetrabenazine (b-HTBZ). a-HTBZ and b-HTBZ are metabolized principally by CYP2D6. Peak plasma concentrations (Cmax) of a-HTBZ and b-HTBZ are reached within 1 to 1 1/2 hours post-dosing. a-HTBZ is subsequently metabolized to a minor metabolite, 9-desmethyl-a-DHTBZ. b-HTBZ is subsequently metabolized to another major circulating metabolite, 9-desmethyl-b-DHTBZ, for which Cmax is reached approximately 2 hours post-dosing. In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine. In vitro experiments in human liver microsomes demonstrate that deutetrabenazine is extensively biotransformed, mainly by carbonyl reductase, to its major active metabolites, alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine, which are subsequently metabolized primarily by CYP2D6, with minor contributions of CYP1A2 and CYP3A4/5, to form several minor metabolites. Biological Half-Life There is interindividual variability in elimination half-life. The elimination half-life of tetrabenazine was 10 hours following intravenous bolus administration. The oral half-lives of its metabolites, α-HTBZ, β-HTBZ and 9-desmethyl-β-DHTBZ, are seven hours, five hours and 12 hours, respectively. Following a single oral dose of 25 mg tetrabenazine, the elimination half-life was approximately 17.5 hours in subjects with hepatic impairment. The half-life of total (α+β)-HTBZ from deutetrabenazine is approximately 9 to 10 hours. alpha-Dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) and 9-desmethyl-beta-dihydrotetrabenazine have half-lives of 7 hours, 5 hours and 12 hours respectively. /Tetrabenazine metabolites/ The half-life of total (alpha+beta)-dihydrotetrabenazine from deutetrabenazine is approximately 9 to 10 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Tetrabenazine is a solid. It is used as adrenergic uptake inhibitor for the treatment of chorea associated with Huntington's disease. Pharmacology studies demonstrate that betrabenzaine reversibly inhibits the activity of vesicular monoamine transporter 2, resulting in depletion of central dopamine. HUMAN STUDIES: Adverse effects are dose and age related and include depression, fatigue, parkinsonism, and somnolence. Neuroleptic malignant syndrome (NMS), a potentially fatal syndrome, has been reported in patients receiving tetrabenazine and other drugs that reduce dopaminergic transmission. Three episodes of overdose occurred in the open-label trials performed in support of registration. Eight cases of overdose have been reported in the literature. The dose of the drug in these patients ranged from 100 mg to 1 g. Adverse reactions associated with overdose include acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor. Completed suicide, attempted suicide, and 6 cases of suicidal ideation were reported in 187 tetrabenazine recipients. Major human metabolite 9-Desmethyl-beta-dihydrotetrabenazine was not clastogenic in an in vitro chromosomal aberration assay in human peripheral blood mononuclear cells in the presence or absence of metabolic activation. ANIMAL STUDIES: No increase in tumors was observed in transgenic mice treated orally with a major human metabolite, 9-desmethyl-beta-dihydrotetrabenazine (20, 100, and 200 mg/kg/day), for 26 weeks. Oral administration of tetrabenazine (5, 15, or 30 mg/kg/day) to female rats prior to and throughout mating, and continuing through day 7 of gestation resulted in disrupted estrous cyclicity at doses greater than 5 mg/kg/day. No effects on mating and fertility indices or sperm parameters (motility, count, density) were observed when male rats were treated orally with tetrabenazine (5, 15, or 30 mg/kg/day. However, because rats dosed with tetrabenazine do not produce 9-desmethyl-beta-dihydrotetrabenazine, a major human metabolite, this study may not have adequately assessed the potential of the drug to impair fertility in humans. Oral administration of 9-desmethyl-beta-dihydrotetrabenazine (8, 15, and 40 mg/kg/day) to pregnant and lactating rats throughout the period of organogenesis produced increases in embryofetal mortality at 15 and 40 mg/kg/day and reductions in fetal body weights at 40 mg/kg/day, which was also maternally toxic. When 9-desmethyl-beta-dihydrotetrabenazine (8, 15, and 40 mg/kg/day) was orally administered to pregnant rats from the beginning of organogenesis through the lactation period, increases in gestation duration, stillbirths, and offspring postnatal mortality (40 mg/kg/day); decreases in pup weights (40 mg/kg/day); and neurobehavioral (increased activity, learning and memory deficits) and reproductive (decreased litter size) impairment (15 and 40 mg/kg/day) were observed. Maternal toxicity was seen at the highest dose. Tetrabenazine and metabolites alpha-dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ), and 9-desmethyl-beta-dihydrotetrabenazine were negative in an in vitro bacterial reverse mutation assay. Tetrabenazine was clastogenic in an in vitro chromosomal aberration assay in Chinese hamster ovary cells in the presence of metabolic activation. a-HTBZ and b-HTBZ were clastogenic in an in vitro chromosome aberration assay in Chinese hamster lung cells in the presence and absence of metabolic activation. In vivo micronucleus assays were conducted in male and female rats and male mice. Tetrabenazine was negative in male mice and rats but produced an equivocal response in female rats. IDENTIFICATION AND USE: Deutetrabenazine is used as adrenergic uptake inhibitor. It is is indicated for the treatment of chorea associated with Huntington's disease (HD) and tardive dyskinesia in adults. HUMAN STUDIES: Overdoses ranging from 100 mg to 1 g have been reported in the literature with tetrabenazine, a closely related vesicular monoamine transporter 2 (VMAT2) inhibitor. The following adverse reactions occurred with overdosing: acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor. Indirect treatment comparison demonstrates that for the treatment of HD chorea, deutetrabenazine has a favorable tolerability profile compared to tetrabenazine. Deutetrabenazine may increase the risk for suicidality in patients with HD. Deutetrabenazine should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Deutetrabenazine and its deuterated alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites were negative in in vitro chromosome aberration assay in human peripheral blood lymphocytes in the presence or absence of metabolic activation. ANIMAL STUDIES: Oral administration of deutetrabenazine (5, 10, or 30 mg/kg/day) to pregnant rats during organogenesis had no clear effect on embryofetal development. Oral administration of deutetrabenazine (doses of 5, 10, or 30 mg/kg/day) to female rats for 3 months resulted in estrous cycle disruption at all doses. Deutetrabenazine and its deuterated alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites were negative in in vitro bacterial reverse mutation assay in the presence or absence of metabolic activation and in the in vivo micronucleus assay in mice. Hepatotoxicity Tetrabenazine has not been associated with rates of serum enzyme elevations greater than occur with placebo therapy, but information on liver test results during therapy is limited and occasional instances of asymptomatic ALT elevations leading to drug discontinuation or dose modification have been reported by the sponsor. In prelicensure pivotal registration trials in several hundred patients, tetrabenazine was not associated with cases of jaundice or hepatitis. Since licensure, there have been no published reports of clinically apparent liver injury, jaundice or hepatitis attributed to tetrabenazine. Thus, clinically apparent liver injury with jaundice due to tetrabenazine must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Tetrabenazine = 82 - 88%; α-HTBZ = 60 - 68%; β-HTBZ = 59 - 63%. At doses ranging from 50 to 200 ng/mL _in vitro_, tetrabenazine protein binding ranged from 82% to 85%, α-HTBZ binding ranged from 60% to 68%, and β-HTBZ binding ranged from 59% to 63%. Similar protein binding pattern is expected for deutetrabenazine and its metabolites. Interactions We herein describe the case of an 81-year-old Japanese woman with neuroleptic malignant syndrome that occurred 36 days after the initiation of combination therapy with tiapride (75 mg/day) and tetrabenazine (12.5 mg/day) for Huntington's disease. The patient had been treated with tiapride or tetrabenazine alone without any adverse effects before the administration of the combination therapy. She also had advanced breast cancer when the combination therapy was initiated. To the best of our knowledge, the occurrence of neuroleptic malignant syndrome due to combination therapy with tetrabenazine and tiapride has not been previously reported. Tetrabenazine should be administered very carefully in combination with other neuroleptic drugs, particularly in patients with a worsening general condition. The efficacy of the dopaminergic stabilizer, pridopidine, in reducing the voluntary and involuntary motor symptoms of Huntington's disease (HD) is under clinical evaluation. Tetrabenazine is currently the only approved treatment for chorea, an involuntary motor symptom of HD; both compounds influence monoaminergic neurotransmission. /The objective of the study was/ to investigate pharmacological interactions between pridopidine and tetrabenazine. Drug-interaction experiments, supplemented by dose-response data, examined the effects of these compounds on locomotor activity, on striatal levels of dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC), and on levels of activity-regulated cytoskeleton-associated (Arc) gene expression in the striatum and frontal cortex of male Sprague-Dawley rats. Haloperidol, a classical dopamine D2 receptor antagonist, was also tested for comparison. Monitoring for 1 hour after co-administration of tetrabenazine 0.64 mg/kg and pridopidine 32 mg/kg revealed a reduction in locomotor activity, measured as distance travelled, in the tetrabenazine treated group, down to 61% vs. vehicle controls (p < 0.001). This was significantly alleviated by pridopidine (distance travelled reached 137% vs. tetrabenazine controls, p < 0.01). In contrast, co-administration of haloperidol 0.12 mg/kg and tetrabenazine produced increased inhibition of locomotor activity over the same period (p < 0.01, 41% vs. tetrabenazine). Co-administration of pridopidine, 10.5 mg/kg or 32 mg/kg, with tetrabenazine counteracted significantly (p < 0.05) and dose-dependently the decrease in frontal cortex Arc levels induced by tetrabenazine 0.64 mg/kg (Arc mRNA reached 193% vs. tetrabenazine mean at 32 mg/kg); this counteraction was not seen with haloperidol. Tetrabenazine retained its characteristic neurochemical effects of increased striatal DOPAC and reduced striatal dopamine when co-administered with pridopidine. Pridopidine alleviates tetrabenazine-induced behavioural inhibition in rats. This effect may be associated with pridopidine-induced changes in cortical activity and may justify clinical evaluation of pridopidine/tetrabenazine combination therapy. The risk for Parkinsonism, neuroleptic malignant syndrome (NMS), and akathisia may be increased by concomitant use of Xenazine and dopamine antagonists or antipsychotics (e.g., chlorpromazine, haloperidol, olanzapine, risperidone, thioridazine, ziprasidone) Potential pharmacologic interaction (serotonin and norepinephrine depletion in the CNS). Concomitant therapy is contraindicated. Clinicians should wait for signs of chorea to re-emerge after discontinuing reserpine before initiating tetrabenazine therapy. At least 20 days should elapse after reserpine discontinuance prior to initiating tetrabenazine therapy. For more Interactions (Complete) data for Tetrabenazine (12 total), please visit the HSDB record page. Austedo is contraindicated in patients currently taking tetrabenazine or valbenazine. Austedo may be initiated the day following discontinuation of tetrabenazine Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence. The risk of parkinsonism, neuroleptic malignant syndrome (NMS), and akathisia may be increased by concomitant use of Austedo and dopamine antagonists or antipsychotics. Austedo is contraindicated in patients taking monoamine oxidase inhibitors (MAOIs). Austedo should not be used in combination with an MAOI, or within 14 days of discontinuing therapy with an MAOI. For more Interactions (Complete) data for Deutetrabenazine (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse iv 150 mg/kg LD50 Mouse sc 400 mg/kg LD50 Mosue ip 250 mg/kg LD50 Mouse oral 550 mg/kg In long-term clinical trials (≥1 year) of patients with hyperkinetic movement disorders, Tetrabenazine (Xenazine) (daily dose 25–100 mg) was associated with common adverse events including depression (28%), anxiety (22%), somnolence (18%), and nausea (15%). Severe adverse events (suicidal ideation, hypotension) occurred in <5% of patients [4] In patients treated with Tetrabenazine (Xenazine) for 6–12 months, no significant changes in liver function (ALT, AST) or renal function (creatinine, BUN) were observed. Plasma protein binding of Tetrabenazine (Xenazine) was 82–88% [5] Abrupt discontinuation of Tetrabenazine (Xenazine) in patients treated for ≥3 months was associated with withdrawal symptoms (agitation, insomnia, rebound hyperkinesia) in 12% of cases. Gradual dose reduction over 2–4 weeks mitigated these effects [6] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Adrenergic Uptake Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Tetrabenazine is included in the database. Xenazine is indicated for the treatment of chorea associated with Huntington's disease. /Included in US product label/ /EXPL THER/ Since levodopa-induced peak dyskinesias (LIDs) may reflect, in part, a disproportionate phasic release of dopamine from synaptic vesicles, we examined the ability of the vesicular depletor tetrabenazine (TBZ) to reduce LIDs in 10 dyskinetic advanced Parkinson's disease (PD) patients. After basal evaluation, the patients received, through a slow titration, oral TBZ twice a day for six weeks (up to 50 mg daily) before being re-assessed after a challenge with levodopa. The primary outcome measure was the change in the Unified Parkinson's Disease Rating Scale (UPDRS) dyskinesia score (items 32 to 34). TBZ was well tolerated. A clear treatment effect on LIDs emerged (up to 45%, p<0.05). In two patients a little worsening of motor performance necessitated an increase of the antiparkinsonian therapy, which did not worsen peak-dose LIDs. The patients experienced a clear benefit in terms of their quality of life. In this open-label pilot study, orally administered TBZ resulted in objective and subjective improvements in LIDs. Larger pharmacological studies are in progress. For more Therapeutic Uses (Complete) data for Tetrabenazine (8 total), please visit the HSDB record page. Adrenergic Uptake Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Dutetrabenazine is included in the database. Austedo is indicated for the treatment of chorea associated with Huntington's disease. /Included in US product label/ Austedo is indicated for the treatment of tardive dyskinesia in adults. /Included in US product label/ /EXPL THER/ Deutetrabenazine, an inhibitor of vesicular monoamine transporter type 2 (VMAT2) depletes presynaptic dopamine and is useful in the treatment of hyperkinetic movement disorders. This study explored the safety, tolerability, and preliminary efficacy of deutetrabenazine in adolescents with moderate-to-severe tics associated with Tourette syndrome (TS). In this open-label study of 12-18-year-old patients with TS-related tics, deutetrabenazine was titrated up to 36 mg/day over 6 weeks to adequately suppress tics without bothersome adverse effects (AEs), followed by maintenance at optimal dose for 2 weeks. An independent blinded rater assessed tic severity using the Yale Global Tic Severity Scale (YGTSS), which was the primary outcome measure. Secondary outcome measures included the TS Clinical Global Impression (TS-CGI) and TS Patient Global Impression of Change (TS-PGIC). Twenty-three enrolled patients received deutetrabenazine and had at least 1 post-baseline YGTSS assessment. The mean (SD [standard deviation]) baseline YGTSS Total Tic Severity Score (TTS) was 31.6 (7.9) and had decreased by 11.6 (8.2) points at week 8, a 37.6% reduction in tic severity (p<0.0001). The TS-CGI score improved by 1.2 (0.81) points (p<0.0001) and the TS-PGIC results at week 8 indicated that 76% of patients were much improved or very much improved compared with baseline. The mean (SD) daily deutetrabenazine dose at week 8 was 32.1 (6.6) mg (range 18-36 mg). One week after withdrawal of deutetrabenazine, the TTS scores increased by 5.6 (8.4) points, providing confirmation of the drug effect. No serious or severe adverse events were reported. The results of this open-label 8-week study suggest that deutetrabenazine is safe and associated with improvement in tic severity in adolescents with TS and troublesome tics. Drug Warnings /BOXED WARNING/ WARNING: DEPRESSION AND SUICIDALITY. Xenazine can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of Xenazine must balance the risks of depression and suicidality with the clinical need for control of chorea. Close observation of patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior should accompany therapy. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician. Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. Xenazine is contraindicated in patients who are actively suicidal, and in patients with untreated or inadequately treated depression. Caution should be exercised in treating patients with tetrabenazine who have a history of depression or prior suicide attempts or ideation since these patients may be at an increased risk for suicidal behavior. Patients who are actively suicidal or those with untreated or inadequately treated depression should not be treated with the drug. Huntington's disease is a progressive disorder characterized by changes in mood, cognition, chorea, rigidity, and functional capacity over time. In a 12-week controlled trial, Xenazine was also shown to cause slight worsening in mood, cognition, rigidity, and functional capacity. Whether these effects persist, resolve, or worsen with continued treatment is unknown. Prior to administering tetrabenazine dosages exceeding 50 mg daily, the manufacturer recommends patients be tested to determine their cytochrome P-450 (CYP) isoenzyme 2D6 status (i.e., poor metabolizers, extensive metabolizers, intermediate metabolizers).1 Drug exposure will be substantially higher (about threefold for alpha-dihydrotetrabenazine (a-HTBZ) and ninefold for beta-dihydrotetrabenazine (beta-HTBZ), both active metabolites) when a dose is given to a poor metabolizer than when given to an extensive metabolizer.1 The manufacturer recommends limiting the dosage of tetrabenazine to 50 mg daily and single doses of the drug to 25 mg in patients who are poor CYP2D6 metabolizers. For more Drug Warnings (Complete) data for Tetrabenazine (22 total), please visit the HSDB record page. /BOXED WARNING/ WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON'S DISEASE. Austedo can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of Austedo must balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician. Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. Austedo is contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression. Huntington's disease is a progressive disorder characterized by changes in mood, cognition, chorea, rigidity, and functional capacity over time. Vesicular monoamine transporter 2 (VMAT2) inhibitors, including deutetrabenazine, may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically reevaluate the need for deutetrabenazine in their patients by assessing the effect on chorea and possible adverse effects, including sedation/somnolence, depression and suicidality, parkinsonism, akathisia, restlessness, and cognitive decline. It may be difficult to distinguish between adverse reactions and progression of the underlying disease; decreasing the dose or stopping the drug may help the clinician to distinguish between the two possibilities. In some patients, the underlying chorea itself may improve over time, decreasing the need for deutetrabenazine. Deutetrabenazine may increase the risk of akathisia, agitation, and restlessness in patients with Huntington's disease and tardive dyskinesia. In a 12-week, double-blind, placebo-controlled trial in Huntington's disease patients, akathisia, agitation, or restlessness was reported by 4% of patients treated with deutetrabenazine, compared to 2% of patients on placebo; in patients with tardive dyskinesia, 2% of patients treated with deutetrabenazine and 1% of patients on placebo experienced these events. Patients receiving deutetrabenazine should be monitored for signs and symptoms of restlessness and agitation, as these may be indicators of developing akathisia. If a patient develops akathisia during treatment with deutetrabenazine, the deutetrabenazine dose should be reduced; some patients may require discontinuation of therapy. A potentially fatal symptom complex sometimes referred to as neuroleptic malignant syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission. While NMS has not been observed in patients receiving deutetrabenazine, it has been observed in patients receiving tetrabenazine (a closely related VMAT2 inhibitor). Clinicians should be alerted to the signs and symptoms associated with NMS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria, rhabdomyolysis, and acute renal failure. The diagnosis of NMS can be complicated; other serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal disorders can present with similar signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology. /Tetrabenazine/ For more Drug Warnings (Complete) data for Deutetrabenazine (14 total), please visit the HSDB record page. Pharmacodynamics Prolongation of the QTc interval has been observed at doses of 50 mg. In rats, it has been observed that tetrabenazine or its metabolites bind to melanin-containing tissues such as the eyes and skin. After a single oral dose of radiolabeled tetrabenazine, radioactivity was still detected in eye and fur at 21 days post dosing. In clinical trials, there was an evidence of clinical effectiveness of deutetrabenazine in improving the symptoms of involuntary movements in patient with tardive dyskinesia by reducing the mean Abnormal Involuntary Movement Scale (AIMS) score. In a randomized, double-blind, placebo-controlled crossover study in healthy male and female subjects, single dose administration of 24 mg deutetrabenazine results in an approximately 4.5 msec mean increase in QTc. Effects at higher exposures to deutetrabenazine or its metabolites have not been evaluated. Deutetrabenazine and its metabolites were shown to bind to melanin-containing tissues including eyes, skin and fur in pigmented rats. After a single oral dose of radiolabeled deutetrabenazine, radioactivity was still detected in eye and fur at 35 days following dosing. Tetrabenazine (Xenazine) exerts its pharmacological effects by inhibiting VMAT-2, which reduces the vesicular storage and subsequent release of monoamine neurotransmitters (dopamine, 5-hydroxytryptamine, norepinephrine) in the central nervous system [2] Tetrabenazine (Xenazine) is indicated for the treatment of hyperkinetic movement disorders (e.g., Huntington’s disease-associated chorea, dystonia) and tardive dyskinesia [4] In MAO-A knockout mice, the anti-aggressive effect of Tetrabenazine (Xenazine) is mediated by reduced dopamine availability in the prefrontal cortex and amygdala [1] Tetrabenazine (Xenazine) does not induce extrapyramidal side effects (e.g., parkinsonism) in humans at therapeutic doses, unlike typical antipsychotics [5] The therapeutic effect of Tetrabenazine (Xenazine) in tardive dyskinesia is correlated with reduced striatal dopamine release, which normalizes abnormal motor circuitry activity [6] |
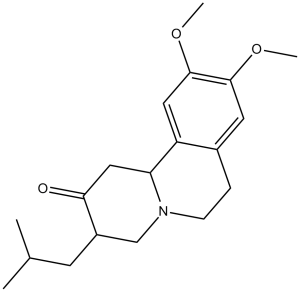
| 分子式 |
C19H27NO3
|
|---|---|
| 分子量 |
317.42
|
| 精确质量 |
317.199
|
| CAS号 |
58-46-8
|
| 相关CAS号 |
Tetrabenazine Racemate;718635-93-9;(+)-Tetrabenazine;1026016-83-0;Tetrabenazine-d6;1392826-25-3;Tetrabenazine mesylate;804-53-5;(+)-Tetrabenazine-d6;1977511-05-9
|
| PubChem CID |
6018
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
448.9±45.0 °C at 760 mmHg
|
| 熔点 |
128-130ºC
|
| 闪点 |
225.3±28.7 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.554
|
| LogP |
3.48
|
| tPSA |
38.77
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
425
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
MKJIEFSOBYUXJB-HOCLYGCPSA-N
|
| InChi Code |
InChI=1S/C19H27NO3/c1-12(2)7-14-11-20-6-5-13-8-18(22-3)19(23-4)9-15(13)16(20)10-17(14)21/h8-9,12,14,16H,5-7,10-11H2,1-4H3/t14-,16-/m0/s1
|
| 化学名 |
(3S,11bS)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one
|
| 别名 |
Tetrabenazine; trade names Nitoman and Xenazin; Ro 1 9569; Ro-1 9569; Ro 1-9569
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1504 mL | 15.7520 mL | 31.5040 mL | |
| 5 mM | 0.6301 mL | 3.1504 mL | 6.3008 mL | |
| 10 mM | 0.3150 mL | 1.5752 mL | 3.1504 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Efficacy of Deutetrabenazine to Control Symptoms of Dysphagia Associated With HD
CTID: NCT04301726
Phase: Phase 1 Status: Unknown status
Date: 2020-07-21