| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Microtubule; tubulin polymerization
|
|---|---|
| 体外研究 (In Vitro) |
Thiocolchicine 对 MCF-7、LoVo、LoVo/DX、A-549 和 BALB/3T3 细胞的 IC50 值分别为 0.01 μM、0.021 μM、0.398 μM、0.011 μM 和 0.114 μM [3]。硫代秋水仙碱(1 nM-100 μM;24-72 小时)在乳腺癌细胞中表现出生长抑制和细胞周期阻断活性之间的相关性。它抑制 MDR CEM-VBL 白血病细胞 (IC50=50 nM) 和多重耐药 (MDR) MCF-7 ADRr 乳腺癌细胞(IC50 分别为 0.6 nM 和 400 nM)的生长。 2]。
|
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD50 intraperitoneal 997 ug/kg Journal of Medicinal Chemistry., 24(636), 1981
|
| 参考文献 |
|
| 其他信息 |
Thiocolchicine is an antimitotic alkaloid that binds to microtubules and inhibits tubulin polymerization and induces apoptosis. (NCI)
Derivatives of colchicine and the bicyclic colchicine analog 2-methoxy-5-(2',3',4'-trimethoxyphenyl)tropone were tested for inhibition of tubulin polymerization. The nature of the tropone substituent had litte effect on the efficacy of the colchicine series, with some exceptions. In contrast, the potency of the bicyclic analogs varied greatly with the tropone substituent. Derivatives of colchicine (I) and its bicyclic analog (II) with varying tropone substituents (R) were prepared and assayed for inhibition of microtubule assembly. Significantly greater variations in potency are observed in the bicyclic series than in the colchicine series.[1] In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and thiocolchicine on three different human cancer cell lines, two of which express the multidrug-resistance (MDR) phenotype. At concentrations from 1 nM to 100 microM, all compounds tested inhibited cancer cell proliferation. The IC50 values indicate that the three fluorinated analogues were the most active compounds, with a similar decreasing order of potency (IDN 5005 > IDN 5079 > IDN 5080) on the two MDR-expressing cell lines, whereas thiocolchicine was the most effective compound on the MDR-negative MDA-MB 231 cells. A strong correlation (r = 0.94; P = 0.004) was found between IC50 values obtained using the two MDR-positive cell lines. Conversely, IC50 values obtained in MDA-MB 231 cells did not show a significant correlation with MDR-positive cell lines, thereby suggesting some difference in the antiproliferative mechanism(s) of colchicine analogues. Cell cycle analysis of the most active analogues in breast cancer cells showed a relationship between cell cycle blocking activity and growth inhibition. The most active agents on the MDR-positive MCF7 ADRr cell line, after 24 h of culture, in terms of cell cycle blocking activity were the three fluorinated analogues. Interestingly, after 72 h, when the cell cycle block subsided, a consistent amount of DNA fragmentation was evident. The extent of cell cycle block, measured as the G2/G1 ratio, was significantly correlated with the apoptosis rate expressed as a percentage of DNA fragmentation on both cell lines, thereby suggesting that a large number of blocked cells underwent the apoptotic pathway.[2] |
| 分子式 |
C22H25NO5S
|
|---|---|
| 分子量 |
415.5026
|
| 精确质量 |
415.145
|
| 元素分析 |
C, 63.59; H, 6.06; N, 3.37; O, 19.25; S, 7.72
|
| CAS号 |
2730-71-4
|
| 相关CAS号 |
Thiocolchicine-d3;1314417-95-2
|
| PubChem CID |
17648
|
| 外观&性状 |
Light yellow to green yellow solid powder
|
| 密度 |
1.27g/cm3
|
| 沸点 |
729.1ºC at 760mmHg
|
| 闪点 |
394.7ºC
|
| 蒸汽压 |
4.12E-21mmHg at 25°C
|
| 折射率 |
1.609
|
| LogP |
3.975
|
| tPSA |
99.16
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
744
|
| 定义原子立体中心数目 |
1
|
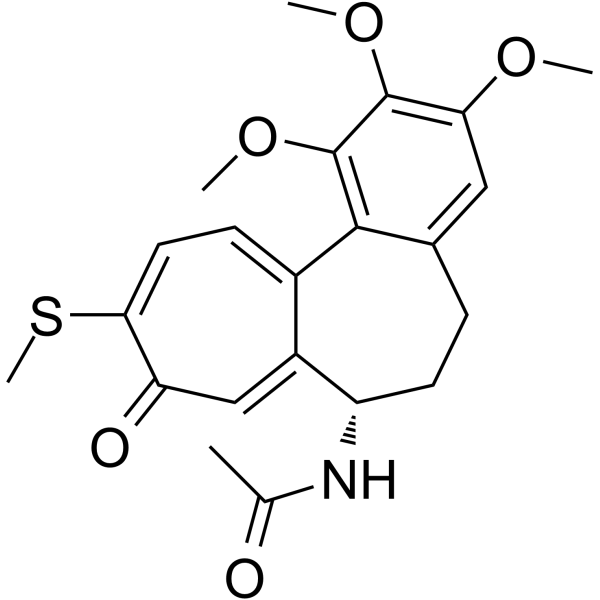
| SMILES |
CC(=O)N[C@H]1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)SC)OC)OC)OC
|
| InChi Key |
CMEGANPVAXDBPL-INIZCTEOSA-N
|
| InChi Code |
InChI=1S/C22H25NO5S/c1-12(24)23-16-8-6-13-10-18(26-2)21(27-3)22(28-4)20(13)14-7-9-19(29-5)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1
|
| 化学名 |
N-[(7S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide
|
| 别名 |
Thiocolchicine; 2730-71-4; Thiocholchicine; Colchicine, 10-thio-; NSC 186301; Colchicine, 10-demethoxy-10-(methylthio)-; EINECS 220-346-8; 10-Demethoxy-10-methylthiocolchicine;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~240.67 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.02 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.02 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4067 mL | 12.0337 mL | 24.0674 mL | |
| 5 mM | 0.4813 mL | 2.4067 mL | 4.8135 mL | |
| 10 mM | 0.2407 mL | 1.2034 mL | 2.4067 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。