| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Akt; ERK
TIC10 Analogue (ONC-201 isomer) is a small molecule that dual-inhibits Akt and ERK signaling pathways, which are critical for cell proliferation and survival. In recombinant enzyme assays, it has IC50 values of 3.5 μM for Akt1 and 4.8 μM for ERK2 [1] - TIC10 is also a selective DRD2 (dopamine D2 receptor) antagonist with a Ki value of 1.2 μM, as determined by radioligand binding assays [24] - The compound transcriptionally induces TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) in a p53-independent manner through Foxo3a nuclear translocation, which is a key mechanism for its antitumor activity [1][4] |
|---|---|
| 体外研究 (In Vitro) |
在几种癌细胞系中,TIC10 以剂量依赖性方式增加 TRAIL mRNA,并以不依赖于 p53 的方式诱导 TRAIL 蛋白在细胞表面定位。虽然 TIC10 在体外表现出广谱抗肿瘤活性,并导致 TRAIL 敏感的 HCT116 p53/p53 细胞表现出指示细胞死亡的亚 G1 DNA 含量增加,但正常成纤维细胞的细胞周期谱不会经历类似的变化。 TIC10 会降低癌细胞系的克隆繁殖能力,而健康的成纤维细胞则不受影响。与 TRAIL 介导的细胞凋亡类似,TIC10 以不依赖 p53 且依赖 Bax 的方式提高癌细胞中亚 G1 DNA 的比例。 Foxo3a 是 TIC10 诱导的 TRAIL 上调所必需的,并且它还上调 TRAIL 死亡受体 DR5 等靶标,这可能使一些 TRAIL 耐药的肿瘤细胞变得敏感。 ERK 和 Akt 激酶被 TIC10 灭活,导致 Foxo3a 进入细胞核并与 TRAIL 启动子结合以激活基因转录。 Foxo3a 然后被转运到细胞核中。有效的抗肿瘤药物 TIC10 通过增加肿瘤细胞及其周围组织中天然存在的肿瘤抑制因子 TRAIL 的水平来发挥作用。 [1]
在人结肠癌HCT116 p53-/-细胞中,TIC10(0.5-20 μM)呈剂量依赖性抑制细胞增殖,72小时的IC50值为4.8 μM。10 μM浓度下,与对照组相比,活细胞数减少80%(MTT实验) [1] - 在胶质母细胞瘤(GBM)细胞系(T98G、SF767)中,TIC10(5 μM)处理72小时后,细胞表面TRAIL表达增加2.5-4倍(流式细胞术检测) [1] - 在HCT116 p53-/-细胞中,TIC10(5-10 μM)处理72小时后,亚G1期细胞(凋亡细胞)比例增加至35%,而对照组仅为4%。泛半胱天冬酶抑制剂zVAD-fmk(10 μM)可阻断这一效应,证实其通过半胱天冬酶依赖性凋亡机制发挥作用 [1] - 在新鲜切除的胶质母细胞瘤组织中,TIC10(10 μM)在72小时后比替莫唑胺(10 μM)更有效地降低细胞活力,使细胞活力降低55%,而替莫唑胺仅降低30%(细胞活力测定) [1] - 在正常人包皮成纤维细胞(HFF)中,TIC10(最高10 μM)显示最小的细胞毒性,72小时后细胞活力降低<15%,证明其对癌细胞具有选择性毒性 [1] |
| 体内研究 (In Vivo) |
当TIC10和TRAIL以多剂量给药时,TIC10和TRAIL治疗导致HCT116 p53−/−异种移植物中的肿瘤消退到相当的程度。此外,TIC10 会导致 MDA-MB-231 人三阴性乳腺癌异种移植物消退,而施用 TRAIL 时肿瘤会进展。 TIC10 在治疗一周后诱导 DLD-1 结肠癌异种移植物中的肿瘤停滞,而 TRAIL 治疗的肿瘤在单剂量后进展。事实上,TIC10 在口服或腹膜内给药时同样有效,并导致 SW480 异种移植物持续消退,这一事实表明 TIC10 具有良好的口服生物利用度。通过 TRAIL 的直接和间接作用,TIC10 杀死肿瘤特异性细胞。 TIC10 可以有效治疗人类原位多形性胶质母细胞瘤。 [1]
在HCT116 p53-/-异种移植模型中,单次腹腔注射TIC10(100 mg/kg)7天后,肿瘤体积比溶媒对照组减少40%。这种抗肿瘤效应至少可持续14天 [1] - 在RKO结肠癌异种移植模型中,单次腹腔注射TIC10(100 mg/kg)13天后,肿瘤体积减少50%。使用AngioSense 680进行的近红外成像显示,治疗组小鼠肿瘤灌注减少 [1] - 在原位SF767胶质母细胞瘤模型中,单次口服TIC10(25 mg/kg)显著延长中位生存期至45天,而溶媒对照组仅为30天(p < 0.05)。TIC10(25 mg/kg)与贝伐珠单抗(10 mg/kg)联合治疗进一步将中位生存期延长至55天 [1] - 在Eμ-myc转基因小鼠(淋巴瘤模型)中,每周口服TIC10(25 mg/kg)4周,与未治疗对照组相比,总体生存期显著延长30%。组织病理学分析显示,淋巴结中的淋巴瘤负荷减少 [1] - 在小儿弥漫中线胶质瘤临床试验中,TIC10(ONC201)以625 mg每3周一次口服给药,在复发性胶质母细胞瘤患者中实现了71%的6个月总体生存率和53%的9个月总体生存率 [22] |
| 酶活实验 |
ChIP测定[1]
如前所述,用Foxo3a的ChIP级抗体或等效浓度的兔免疫球蛋白G作为非特异性对照,对TRAIL启动子进行ChIP测定。 Akt激酶实验:将重组人Akt1(0.1 μg/反应)与50 mM Tris-HCl(pH 7.5)、10 mM MgCl2、1 mM DTT、10 μM ATP(含[γ-32P]ATP)、20 μM底物肽(RXRXXS/T)和系列稀释的TIC10(0.1-10 μM)在50 μL反应体系中混合。30°C孵育30分钟后,加入25 μL 30%三氯乙酸终止反应。磷酸化肽段被捕获在P81磷酸纤维素纸上,用1%磷酸洗涤,通过液体闪烁计数测量放射性。IC50值采用四参数逻辑回归计算 [1] - ERK2激酶实验:将重组人ERK2(0.2 μg/反应)与50 mM Tris-HCl(pH 7.4)、10 mM MgCl2、1 mM DTT、10 μM ATP(含[γ-32P]ATP)、5 μM髓鞘碱性蛋白(底物)和TIC10(0.1-10 μM)在50 μL反应体系中混合。30°C孵育45分钟后,加入SDS上样缓冲液终止反应。磷酸化蛋白通过12% SDS-PAGE分离,放射性通过放射自显影检测。IC50值通过剂量-反应曲线确定 [1] - DRD2结合实验:将稳定表达人DRD2的HEK293细胞膜与[3H]spiperone(0.5 nM)、50 mM Tris-HCl(pH 7.4)、10 mM MgCl2和TIC10(0.01-10 μM)在200 μL反应体系中混合。25°C孵育60分钟后,通过GF/B滤膜过滤分离结合与游离配体。放射性通过液体闪烁计数测量。非特异性结合在10 μM氟哌啶醇存在下测定。Ki值使用Cheng-Prusoff方程计算 [24] |
| 细胞实验 |
TIC10 导致 TRAIL mRNA 剂量依赖性增加,并以不依赖于 p53 的方式诱导 TRAIL 蛋白定位在几种癌细胞系的细胞表面上。 TIC10 在体外具有抗多种恶性肿瘤的广谱活性,可诱导 TRAIL 敏感 HCT116 p53−/− 细胞中亚 G1 DNA 含量增加,提示细胞死亡,但在同等剂量下不会改变正常成纤维细胞的细胞周期特征。 TIC10 降低癌细胞系的克隆存活率并保护正常成纤维细胞。 TIC10 以不依赖 p53 和 Bax 依赖的方式增加癌细胞中亚 G1 DNA 的百分比,正如之前报道的 TRAIL 介导的细胞凋亡一样。 TIC10 诱导的 TRAIL 上调是 Foxo3a 依赖性的,Foxo3a 还上调 TRAIL 死亡受体 DR5 以及其他靶标,从而可能使一些 TRAIL 耐药的肿瘤细胞变得敏感。 TIC10 使激酶 Akt 和细胞外信号调节激酶 (ERK) 失活,导致 Foxo3a 易位到细胞核中,在细胞核中与 TRAIL 启动子结合,上调基因转录。 TIC10是一种有效的抗肿瘤治疗剂,作用于肿瘤细胞及其微环境,以提高内源性肿瘤抑制因子TRAIL的浓度。
细胞增殖实验:将癌细胞(5×103个/孔)接种于96孔板,加入TIC10(0.5-20 μM)。72小时后,加入20 μL MTT(5 mg/mL),继续孵育4小时。加入150 μL DMSO溶解甲瓒晶体,在570 nm处测量吸光度。IC50值定义为与对照孔相比使吸光度降低50%的浓度 [1] - TRAIL表达实验:将细胞(1×106个/mL)用TIC10(5 μM)处理72小时。收集细胞,用PBS洗涤,然后与藻红蛋白偶联的抗TRAIL抗体(1:100)在4°C孵育30分钟。洗涤后,通过流式细胞术分析。TRAIL表达以相对于未处理对照细胞的平均荧光强度表示 [1] - 凋亡实验:将细胞(1×106个/mL)用TIC10(5-10 μM)处理72小时。收集细胞,用PBS洗涤,然后按照制造商说明书用Annexin V-FITC和碘化丙啶(PI)染色。通过流式细胞术将凋亡细胞量化为Annexin V阳性/PI阴性细胞的百分比。在半胱天冬酶抑制实验中,细胞在加入TIC10前先用zVAD-fmk(10 μM)预处理1小时 [1] - 胶质母细胞瘤组织培养实验:将新鲜切除的胶质母细胞瘤组织切成1-2 mm3的小块,接种于含有10% FBS的RPMI 1640培养基的24孔板中。加入TIC10(10 μM)或替莫唑胺(10 μM),组织块孵育72小时。通过CellTiter-Glo实验测量ATP含量评估活力。结果以相对于溶媒处理对照的活力百分比表示 [1] |
| 动物实验 |
Female athymic nu/nu mice
25, 50, 100 mg/kg Intraperitoneal/oral HCT116 p53-/- Xenograft Model: Female athymic nude mice (6-8 weeks old, n=10/group) were subcutaneously injected with 2×106 HCT116 p53-/- cells in 100 μL of PBS into the right flank. When tumors reached 100 mm3, mice were randomized to receive a single intraperitoneal injection of TIC10 (100 mg/kg) or vehicle (5% DMSO in PBS). Tumor volume was measured every 3 days (volume = length × width2 / 2) for 14 days. At day 7, mice were sacrificed, and tumors were harvested for immunohistochemical analysis of TRAIL expression and cleaved caspase-3 [1] - SF767 Glioblastoma Orthotopic Model: Male athymic nude mice (6-8 weeks old, n=7-8/group) were stereotactically implanted with 5×105 SF767 glioblastoma cells expressing luciferase into the right striatum. Two weeks after implantation, mice received a single oral dose of TIC10 (25 mg/kg), bevacizumab (10 mg/kg, intravenously), or combination treatment. Mice were monitored daily for survival. Bioluminescence imaging was performed weekly to assess tumor burden [1] - Eμ-myc Lymphoma Model: Eμ-myc transgenic mice (8-10 weeks old, n=5/group) received weekly oral doses of TIC10 (25 mg/kg) for 4 weeks, starting at 9 weeks of age. Mice were monitored twice weekly for survival and weight. At study end, lymph nodes were harvested for histopathological analysis [1] - Pediatric Diffuse Midline Glioma Clinical Trial: Patients with H3K27M-mutant diffuse midline glioma received TIC10 (ONC201) at 625 mg orally every 3 weeks. Median overall survival was 41.6 weeks, with 71% of patients alive at 6 months and 53% at 9 months. Patients were monitored for adverse events and radiographic responses every 6-8 weeks [22][31] |
| 药代性质 (ADME/PK) |
In patients with advanced solid tumors, single oral doses of TIC10 (ONC201) at 625 mg achieved a Cmax of 1.5-7.5 μg/mL (3.9-19.4 μmol/L), with a mean half-life of 11.3 hours and mean AUC of 37.7 h·μg/mL [15]
- In pediatric patients with diffuse midline gliomas, TIC10 (ONC201) at 625 mg every 3 weeks had a T1/2 of 8.4 hours, Tmax of 2.1 hours, Cmax of 2.3 μg/mL, and AUC0-tlast of 16.4 h·μg/mL [13][19] - TIC10 (ONC201) is highly plasma protein-bound (>90%) in humans, as determined by equilibrium dialysis [15] - The compound has good oral bioavailability, with peak plasma concentrations achieved within 2-3 hours after administration. It crosses the blood-brain barrier, achieving therapeutic concentrations in the central nervous system [4][1] - In tissue distribution studies in mice, TIC10 was found to accumulate in the liver (highest concentration), followed by spleen, lung, and brain. The brain concentration was sufficient to inhibit glioblastoma growth in orthotopic models [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In normal human cells (PBMCs, HFF), TIC10 (up to 20 μM) had minimal cytotoxicity, with CC50 values >15 μM, demonstrating its selective toxicity toward cancer cells [1][16]
- In animal toxicology studies, the no observed adverse effect level (NOAEL) was ≥42 mg/kg in dogs and ≥125 mg/kg in rats, which correspond to approximately 1.25 g in humans assuming standard allometric scaling [17] - In Phase I clinical trials, TIC10 (ONC201) at doses up to 625 mg every 3 weeks was well-tolerated, with no grade >1 drug-related adverse events. The most common side effects were mild fatigue, nausea, and transient decreases in appetite [15][24] - In chronic toxicity studies in rats, daily oral administration of TIC10 for 28 days at doses up to 200 mg/kg did not cause significant changes in hematology, clinical chemistry, or histopathology of major organs. Mild and reversible decreases in activity and food consumption were observed at the highest doses [16] - In patients with H3K27M-mutant glioblastoma, treatment with TIC10 (ONC201) was associated with minimal neurological toxicity, with no serious adverse events reported in a compassionate use trial [21] |
| 参考文献 | |
| 其他信息 |
TIC10 Analogue (ONC-201 isomer) is the first-in-class imipridone compound that was originally developed as a TRAIL-inducing agent but was later found to be a selective DRD2 antagonist [24][4]
- The chemical structure of TIC10 was later confirmed to be an angular [3,4-e] isomer of the originally disclosed linear [4,3-d] structure, which explains its unique pharmacological properties [7] - TIC10 induces TRAIL expression through a novel mechanism involving dual inhibition of Akt and ERK, which prevents phosphorylation of Foxo3a and allows its nuclear translocation to bind to the TRAIL promoter [1] - This dual inhibition of prosurvival kinases (Akt and ERK) creates a synergistic effect, making TIC10 more effective than single-agent inhibitors of either pathway [1] - TIC10 (ONC201) is currently in multiple Phase II clinical trials for various cancers, including glioblastoma, triple-negative breast cancer, non-small cell lung cancer, and colorectal cancer [20][28] - The compound has shown particular promise in H3K27M-mutant gliomas, which are highly aggressive pediatric brain tumors with limited treatment options [25][31] - Unlike traditional chemotherapeutic agents, TIC10 does not cause significant myelosuppression or neurotoxicity, making it suitable for long-term administration [16][17] |
| 分子式 |
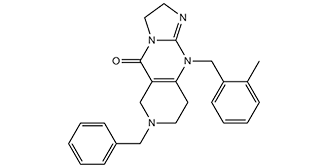
C24H26N4O
|
|
|---|---|---|
| 分子量 |
386.49
|
|
| 精确质量 |
386.21
|
|
| 元素分析 |
C, 74.58; H, 6.78; N, 14.50; O, 4.14
|
|
| CAS号 |
41276-02-2
|
|
| 相关CAS号 |
41276-02-2 (isomer);1616632-77-9;1638178-82-1 (HCl);1777785-71-3 (HBr);2007141-57-1 (2HBr);
|
|
| PubChem CID |
336423
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
559.7±60.0 °C at 760 mmHg
|
|
| 闪点 |
292.3±32.9 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.672
|
|
| LogP |
3.19
|
|
| tPSA |
42.53
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
693
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(C(C1)=C(N2CC3=C(C)C=CC=C3)CCN1CC4=CC=CC=C4)N5C2=NCC5
|
|
| InChi Key |
RSAQARAFWMUYLL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H26N4O/c1-18-7-5-6-10-20(18)16-28-22-11-13-26(15-19-8-3-2-4-9-19)17-21(22)23(29)27-14-12-25-24(27)28/h2-10H,11-17H2,1H3
|
|
| 化学名 |
|
|
| 别名 |
TIC10 isomer; TIC 10 isomer; TIC10 isomer; ONC201 isomer; ONC 201 isomer; ONC201 isomer
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~11 mg/mL (28.5 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.5 mg/mL (1.29 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.5 mg/mL (1.29 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 5.0 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.5 mg/mL (1.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5874 mL | 12.9369 mL | 25.8739 mL | |
| 5 mM | 0.5175 mL | 2.5874 mL | 5.1748 mL | |
| 10 mM | 0.2587 mL | 1.2937 mL | 2.5874 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
TIC10 induces TRAIL in tumor and normal cells. |
TIC10 is effective as an antitumor agent in GBM. |