| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
WT ALK (IC50 = 1.01 nM); ALK(L1196M) (IC50 = 1.08 nM); ALK(G1202R) (IC50 = 1.26 nM); Trk receptor; ROS1
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:TPX-0005 是一种口服有效的 ATP 竞争性抑制剂,针对 ALK、ROS1、TRKA、TRKB 和 TRKC 重组激酶及其相应的临床耐药突变体。TPX-0005 在伤口愈合试验中抑制 H2228 细胞迁移,效果类似萨拉卡替尼的活性。它不仅可以抑制野生型和广谱突变型 ALK,还可以通过抑制 SRC 克服原发性耐药并抑制转移特征。激酶测定:TPX-0005 是一种新型、合理设计的高效 ALK/ROS1/TRK 抑制剂,对 SRC、WT ALK、ALK G1202R 和 ALK L1196M 的 IC50 分别为 5.3 nM、1.01 nM、1.26 nM 和 1.08 nM 。它具有潜在的抗癌活性。它通过强烈抑制 EML4-ALK (IC50 13 nM) 和 SRC 底物桩蛋白 (IC50 107 nM) 的磷酸化,有效克服了这种主要耐药性(细胞增殖测定中 IC50 100 nM)。 PX-0005 在伤口愈合试验中抑制 H2228 细胞迁移,其活性与萨拉卡替尼相似。总体而言,TPX-0005 具有非常有利的特性,能够克服多种 ALK 耐药机制,包括二次突变、旁路信号激活、EMT,并值得临床研究细胞测定:TPX-0005 也是一种有效的 SRC 抑制剂 (IC50 5.3 nM)。 H2228 肺癌细胞系中 SRC 激酶活性升高,导致细胞增殖测定中对克唑替尼 (IC50 1200 nM) 和色瑞替尼 (IC50 1000 nM) 产生耐药性。 TPX-0005 通过强烈抑制 EML4-ALK (IC50 13 nM) 和 SRC 底物桩蛋白 (IC50 107 nM) 以及其他下游信号传导靶标的磷酸化,有效克服了这种主要耐药性(细胞增殖测定中 IC50 100 nM)。 TPX-0005 在伤口愈合试验中抑制 H2228 细胞迁移,其活性与萨拉卡替尼相似。
|
||
| 体内研究 (In Vivo) |
在患者来源的异种移植肿瘤模型中,TPX-0005 治疗可导致含有致癌 ALK、ROS1 和 TRKC 融合的肿瘤显着消退。此外,在一系列小鼠异种移植肿瘤模型中,TPX-0005不仅在含有野生型致癌靶点的肿瘤中表现出显着的抗肿瘤活性,而且通过抑制靶点磷酸化,在含有溶剂前沿突变的癌基因的肿瘤中也表现出显着的抗肿瘤活性。
Repotrectinib抑制神经母细胞瘤异种移植物模型中的肿瘤生长[3] 为了进一步研究Repotrectinib/瑞普替尼在神经母细胞瘤中的作用,我们采用了小鼠异种移植物模型。皮下注射CLB-BAR神经母细胞瘤细胞,所得肿瘤用瑞普替尼(20mg/kg,每日两次)、克唑替尼(80mg/kg,每日一次)或赋形剂对照治疗。在14天的治疗过程中,接受瑞普替尼治疗的动物肿瘤体积略有增加(图4a)。瑞普替尼和克唑替尼的肿瘤生长抑制(TGI)值分别为87.07%和66.4%(图4a)。14天后,在瑞普替尼药物释放后,肿瘤恢复生长(图4a)。第6天后,载体对照组的肿瘤继续生长,与瑞普替尼治疗相比显著增加(p=0.008)(图4a)。正如预期的那样,克唑替尼显示出与先前报道一致的抗肿瘤活性。在第14天,瑞普替尼组和克唑替尼组的肿瘤体积和重量均显著降低,但克唑替尼比瑞普替尼更有效地抑制肿瘤生长(图4a)。除了有效抑制肿瘤生长外,接受雷普替尼治疗的动物体重也有所增加,在14天的实验中体重明显增加(第14天p<0.0001)(图4b)。 |
||
| 酶活实验 |
TPX-0005 是一种新型、合理设计的高效 ALK/ROS1/TRK 抑制剂,对 SRC、WT ALK、ALK G1202R 和 ALK L1196M 的 IC50 值为 5.3 nM、1.01 nM、1.26 nM 和 1.08 nM。那个订单。它可能具有抗癌特性。通过显着抑制 EML4-ALK (IC50 13 nM) 和 SRC 底物桩蛋白 (IC50 107 nM) 的磷酸化,它成功克服了这种主要耐药性(细胞增殖测定中 IC50 100 nM)。在伤口愈合试验中,PX-0005 抑制 H2228 细胞迁移,其活性与 saracatinib 相当。综合考虑,TPX-0005 具有非常有前途的特性,能够克服多种 ALK 耐药机制,例如二次突变、旁路信号激活和 EMT。因此,值得进一步的临床研究。
抑制神经母细胞瘤细胞系中的ALK活性[3] 将CLB-BAR和CLB-GE细胞铺在10cm培养皿中,并如前所述用200或300nM的repotrectinib或克里唑替尼处理。处理1小时后收集细胞裂解物,通过BCA测定蛋白质浓度。通过蛋白质印迹分析蛋白质裂解物,并使用ECLTM Prime蛋白质印迹试剂进行可视化。用0.5M NaOH剥离每层初级磷酸抗体膜30分钟,并重新印迹总蛋白。β-actin用于验证样品加载的均匀性。实验一式三份。图像使用Adobe Photoshop CS6裁剪,最终版本使用Illustrator CS6完成。 PC-12细胞ALK磷酸化IC50[3] 如前所述,用ALK突变体构建体或指定的野生型ALK构建体对细胞进行瞬时转染。通过测序确认了构建体。简要地,在Amaxa电穿孔器中,使用100µL Ingenio电穿孔溶液和0.75μg突变ALK构建体或1.5μg野生型变体对3×106个细胞进行电穿孔。将四次转染合并到10.5mL的最终体积中,每孔将500µL镀入24孔板中。48小时后,用连续稀释的repotrectinib或克唑替尼处理细胞4小时。收集细胞裂解物并通过免疫印迹进行分析。使用Image Studio Lite测定肌动蛋白、磷酸化ALK-Y1604和泛ALK带强度,肌动蛋白用于磷酸化ALG-Y1604的归一化。进行pan-ALK以证实等载荷。使用Adobe Photoshop CS6裁剪图像并调整对比度。ALK磷酸化的IC50定义为相对于未处理的细胞,导致ALK-Y1604磷酸化水平达到50%的药物浓度。 神经突起生长测定[3] 将突变型(0.75μg)或野生型(1.5μg)ALK构建体和pEGFPN1(0.5μg)共转染到2×106个PC-12细胞中。转染后,将细胞稀释在7.5mL培养基中,混合并将300µL接种到24孔板中。第二天,用200 nMrepotrectinib或250 nM克唑替尼处理细胞,用1µg/mL的ALKAL111,50刺激野生型ALK。转染后48小时分析神经突起生长情况。用Zeiss Axiovert 40 CFL显微镜测定神经突起的形成,携带细胞体两倍大小神经突起的GFP阳性细胞被认为是阳性的。实验一式三份。 |
||
| 细胞实验 |
TPX-0005 也是一种有效的 SRC 抑制剂 (IC50 5.3 nM)。在细胞增殖测试中,H2228 肺癌细胞系中 SRC 激酶活性的增加导致对克唑替尼 (IC50 1200 nM) 和色瑞替尼 (IC50 1000 nM) 的耐药性。通过强烈抑制 EML4-ALK (IC50 13 nM) 和 SRC 底物桩蛋白 (IC50 107 nM) 以及其他下游信号传导靶标的磷酸化,TPX-0005 有效克服了这种主要耐药性(细胞增殖测定中 IC50 100 nM) )。与 saracatinib 类似,TPX-0005 在伤口愈合试验中抑制 H2228 细胞迁移。
增殖试验[3] 将神经母细胞瘤细胞系接种到48孔板中,以在治疗时实现30-40%的融合Repotrectinib和克唑替尼溶解在DMSO中,并在加入前新鲜制备。用于增殖试验的瑞普替尼和克唑替尼的浓度分别为50、100、200、300、400和500 nM。DMSO的量不超过总培养基体积的0.1%。将板放置在Incucyte中,每24小时拍摄16张图像/孔,持续5天。每个实验重复三次,重复三次。使用相位对比通道的10倍放大物镜拍摄图像,并使用Incucyte活细胞成像系统进行处理和分析。通过选择基本分析仪、相位对比通道和选择6-8个代表性图像来创建分析定义。调整分割和最小面积(µm2)过滤器,以实现除碎片外的最大细胞检测。对每个细胞系分别进行分析定义,并将这些特定参数用于每个细胞系组中的所有图像。 细胞凋亡测定[3] 将细胞接种在6孔板中,用指定浓度的repotrectinib或克里唑替尼处理24小时。对于蛋白质印迹,使用RIPA缓冲液(50 mM Tris-HCl pH 7.4,1%NP40,150mM氯化钠,2 mM EDTA、0.1%SDS、1x磷酸STOP、1x完全不含EDTA)和蛋白质浓度用Pierce®BCA蛋白质测定试剂盒测定。用PARP抗体对样本进行免疫印迹,该抗体可识别全长和切割的PARP1。在三个独立的实验中,使用肌动蛋白对切割的PARP1进行归一化。在Odyssey Fc系统中,PARP1和肌动蛋白的信号与immobilon Forte Western HRP底物同时可视化,使用Image Studio Lite软件确定条带强度。流式细胞术用于分析用膜联蛋白V和碘化丙啶染色的细胞,作为PARP切割的补充检测。根据制造商的方案(死细胞凋亡试剂盒)在处理后收集细胞并染色,并在使用LSRII流式细胞仪进行分析之前通过细胞过滤器盖将其沉积在5 mL管中。使用FlowJo v9.6软件进行数据分析。图像处理是使用Adobe Photoshop CS6和Illustrator C6S完成的。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The geometric mean (CV%) of repotrectinib steady state peak concentration (Cmax,ss) is 713 (32.5%) ng/mL and the area under the time concentration curve (AUC0-24h,ss) is 7210 (40.1%) ng•h/mL following the approved recommended twice daily dosage in patients with cancer. Repotrectinib Cmax and AUC0-inf increases approximately dose-proportional (but less than linear with estimated slopes of 0.78 and 0.70, respectively) over the single dose range of 40 mg to 240 mg (0.25 to 1.5 times the approved recommended dosage). Steady-state PK was time-dependent with an autoinduction of CYP3A4. Steady-state is achieved within 14 days of daily administration of 160 mg. The geometric mean (CV%) absolute bioavailability of repotrectinib is 45.7% (19.6%). Peak repotrectinib concentration occurred at approximately 2 to 3 hours post a single oral dose of 40 mg to 240 mg (0.25 to 1.5 times the approved recommended dosage) under fasted conditions. No clinically significant differences in repotrectinib pharmacokinetics were observed in patients with cancer following administration of a high-fat meal (approximately 800-1000 calories, 50% fat). Following a single oral 160 mg dose of radiolabeled repotrectinib, 4.84% (0.56% as unchanged) was recovered in urine and 88.8% (50.6% unchanged) in feces. The geometric mean (CV%) apparent volume of distribution (Vz/F) was 432 L (55.9%) in patients with cancer following a single 160 mg oral dose of repotrectinib. The geometric mean (CV%) apparent oral clearance (CL/F) was 15.9 L/h (45.5%) in patients with cancer following a single 160 mg oral dose of repotrectinib. Metabolism / Metabolites Repotrectinib is primarily metabolized by CYP3A4 followed by secondary glucuronidation. Biological Half-Life The repotrectinib mean terminal half-life is approximately 50.6 h for patients with cancer following a single dose. The steady-state repotrectinib terminal half-life is approximately 35.4 h for patients with cancer. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure trials of repotrectinib as therapy of ROS1-positive NSCLC, liver test abnormalities were frequent but usually mild-to-moderate in severity and self-limited in duration. ALT elevations arose in 34% and AST in 40% and were above 5 times the ULN in 3.1% and 1.9% of the 264 patients treated in the major registration trial. The median time to onset of aminotransferase elevations was 15 days (range 1 day to 1 year). Dose interruptions for ALT abnormalities were done in 1.1% of patients but permanent discontinuations were rare. There were no enzyme elevations with jaundice or symptoms and no life threatening or fatal instances of liver injury. Since approval and clinically availability of repotrectinib, there have been no published case reports of clinically apparent liver injury with jaundice, but clinical experience with its use has been limited. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of repotrectinib during breastfeeding. Because repotrectinib is 94.5% bound to plasma proteins, the amount in milk is likely to be low and oral bioavailability is less than 50%; however, the drug’s half-life is about 50 hours in adults. The manufacturer recommends that breastfeeding be discontinued during repotrectinib therapy and for 10 days after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Repotrectinib binding to plasma protein was 95.4% in vitro. The blood-to-plasma ratio was 0.56 in vitro. |
||
| 参考文献 | |||
| 其他信息 |
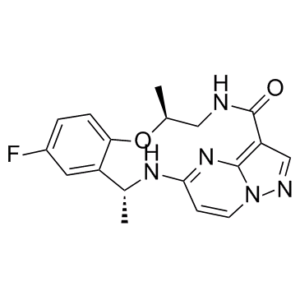
Repotrectinib is an azamacrocycle with formula C18H18FN5O2. It is a tyrosine kinase inhibitor (highly potent against ROS1, TRKA-C, and ALK) used for the treatment of locally advanced or metastatic ROS1-positive non-small cell lung cancer. It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an antineoplastic agent. It is a member of monofluorobenzenes, a pyrazolopyrimidine, a cyclic ether, a secondary carboxamide and an azamacrocycle.
Repotrectinib is a next-generation tyrosine kinase inhibitor (TKI) specifically designed to address resistance in the treatment of non-small cell lung cancer (NSCLC), specifically due to mutations in the ROS1 gene. ROS1 mutations are one of the defined oncogenic drives of NSCLC, and the solvent-front mutation ROS1 G2032R is responsible for 50 to 60% of [crizotinib]-resistant cases. Repotrectinib possesses a compact macrocyclic structure that both limits adverse interactions with resistance mutation hotspots and targets mutations in the solvent-front region. Although resistance to multiple TKI has been reported, including [crizotinib], [lorlatinib], [taletrectinib], and [entrectinib], there has been no reported case of repotrectinib resistance. On November 15th, 2023, the FDA approved repotrectinib under the brand name Augtyro for the treatment of locally advanced or metastatic ROS1-Positive NSCLC. This approval is based on favorable results from the TRIDENT-1 study, where the objective response rate was 79% in TKI-naive patients and 38% in TKI-pretreated patients respectively. Repotrectinib is a Kinase Inhibitor. The mechanism of action of repotrectinib is as a Proto-Oncogene Tyrosine-Protein Kinase ROS1 Inhibitor, and Tropomyosin Receptor Tyrosine Kinase A Inhibitor, and Tropomyosin Receptor Tyrosine Kinase B Inhibitor, and Tropomyosin Receptor Tyrosine Kinase C Inhibitor, and Cytochrome P450 3A4 Inducer. Repotrectinib is an orally available inhibitor of multiple kinases, including the receptor tyrosine kinase anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1), the neurotrophic tyrosine receptor kinase (NTRK) types 1, 2 and 3, the proto-oncogene SRC, and focal adhesion kinase (FAK), with potential antineoplastic activity. Upon oral administration, repotrectinib binds to and inhibits wild-type, point mutants and fusion proteins of ALK, ROS1, NTRK1-3, SRC, FAK and, to a lesser extent, other kinases. Inhibition of these kinases leads to the disruption of downstream signaling pathways and the inhibition of cell growth of tumors in which these kinases are overexpressed, rearranged or mutated. Drug Indication Repotrectinib is indicated for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC). Treatment of all conditions included in the category of malignant neoplasms (except haematopoietic neoplasms) Mechanism of Action Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 (ROS1) and of the tropomyosin receptor tyrosine kinases (TRKs) TRKA, TRKB, and TRKC. In summary, we show that repotrectinib abrogates ALK activity in in vitro biochemical assays, in a manner comparable to crizotinib. However, repotrectinib is superior to crizotinib in abrogating xenograft tumor growth, likely due to its pharmacology properties, and also perhaps reflecting that repotrectinib is a potent inhibitor with a broader target kinase range. Immunostaining of the tumor material showed a significant increase in CD31 for both ALK inhibitors compared to the control group, indicating increased density of blood vessels. The increase of CD31-positive vessels could be in part due to the overall decrease in tumor volume and lead to a perceived increase of expression of CD31-positive vessels. However, we also observe an increase of the pericyte marker desmin in repotrectinib treated tumors, as shown in Fig. 5, indicating an increase in pericyte number. The increase in desmin would suggest that it is not simply tumor shrinkage that leads to an increase of CD31-positive cells. Instead, the increased desmin suggests that tumor stress by ALK TKI treatment leads to hypoxia and subsequent angiogenesis and the recruitment of pericytes that bring about the overall increase of CD31-positive vessels. Increased CD31 levels have been noted before in treated tumors and have been considered to reflect changing architecture in the tumor or an endothelial cell response to the therapeutic challenge. Altogether, these data suggest that upon treatment with the recently described ALK TKI repotrectinib, growth of ALK-driven neuroblastoma cells and xenografts are inhibited, suggesting that repotrectinib should be further explored in a neuroblastoma setting.[3] |
| 分子式 |
C18H18FN5O2
|
|---|---|
| 分子量 |
355.37
|
| 精确质量 |
355.144
|
| 元素分析 |
C, 60.84; H, 5.11; F, 5.35; N, 19.71; O, 9.00
|
| CAS号 |
1802220-02-5
|
| 相关CAS号 |
1802220-02-5; 2058227-19-1
|
| PubChem CID |
135565923
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.694
|
| LogP |
1.71
|
| tPSA |
80.6
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
524
|
| 定义原子立体中心数目 |
2
|
| SMILES |
FC1C=CC2=C(C=1)[C@@H](C)NC1C=CN3C(=C(C=N3)C(NC[C@H](C)O2)=O)N=1
|
| InChi Key |
FIKPXCOQUIZNHB-WDEREUQCSA-N
|
| InChi Code |
InChI=1S/C18H18FN5O2/c1-10-8-20-18(25)14-9-21-24-6-5-16(23-17(14)24)22-11(2)13-7-12(19)3-4-15(13)26-10/h3-7,9-11H,8H2,1-2H3,(H,20,25)(H,22,23)/t10-,11+/m0/s1
|
| 化学名 |
(3R,11S)-6-fluoro-3,11-dimethyl-10-oxa-2,13,17,18,21-pentazatetracyclo[13.5.2.04,9.018,22]docosa-1(21),4(9),5,7,15(22),16,19-heptaen-14-one
|
| 别名 |
Ropotrectinib; TPX0005; 1802220-02-5; Ropotrectinib; Augtyro; 08O3FQ4UNP; Repotrectinib [USAN]; repotrectinibum; TPX-0005; TPX 0005; Augtyro
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.03 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8140 mL | 14.0698 mL | 28.1397 mL | |
| 5 mM | 0.5628 mL | 2.8140 mL | 5.6279 mL | |
| 10 mM | 0.2814 mL | 1.4070 mL | 2.8140 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04094610 | Recruiting | Drug: Oral repotrectinib (TPX-0005) |
Lymphoma Primary CNS Tumors |
Turning Point Therapeutics, Inc. | March 12, 2020 | Phase 1 Phase 2 |
| NCT05004116 | Recruiting | Drug: Irinotecan and temozolomide Drug: Repotrectinib |
Advanced Cancer Metastatic Solid Tumor |
Memorial Sloan Kettering Cancer Center |
August 9, 2021 | Phase 1 Phase 2 |
| NCT04772235 | Recruiting | Drug: Repotrectinib Drug: Osimertinib |
Nsclc | Instituto Oncológico Dr Rosell | February 11, 2022 | Phase 1 |
| NCT05828303 | Recruiting | Drug: TPX-0005 Drug: Digoxin |
Advanced Solid Tumor Metastatic Solid Tumor |
Turning Point Therapeutics, Inc. | July 28, 2022 | Phase 1 |
| NCT03093116 | Recruiting | Drug: Oral repotrectinib (TPX-0005) |
Locally Advanced Solid Tumors Metastatic Solid Tumors |
Turning Point Therapeutics, Inc. | February 27, 2017 | Phase 1 Phase 2 |