| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
全身(腹膜内)注射高剂量(600 或 1200 mg/kg)氨己烯酸后,发现癫痫阈值显着升高。双侧黑质前部或后部网状部显微注射氨己烯酸(10μg)也改善了癫痫阈值,但不如全身治疗那么显着。局部递送至丘脑底核 (STN) 比黑内酯或氨己烯酸全身治疗更显着地增加癫痫阈值 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
在 Caco-2 和 MDCK 细胞中,30 mM 氨己烯酸使牛磺酸的摄取分别减少 34% 和 53%。 Caco-2 细胞中氨己烯酸的吸收具有浓度依赖性,并且在中性 pH 下可饱和,Km 值为 27 mM。在肾脏和肠道细胞培养模型中,氨己烯酸会降低牛磺酸的吸收[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption following oral administration is essentially complete. The Tmax is approximately 2.5 hours in infants (5m - 2y) and 1 hour in all other age groups. Approximately 95% of the drug is eliminated in the urine within 72 hours of administration, of which ~80% is unchanged parent drug. Vigabatrin is widely distributed throughout the body with a mean steady-state volume of distribution of 1.1 L/kg. The oral clearance of vigabatrin is 2.4 L/h for infants (5m - 2y), 5.1 L/h for children (3y - 9y), 5.8 L/h for adolescents (10y - 16y), and 7 L/h for adults. Drug transporters in various tissues, such as intestine, kidney, liver and brain, are recognized as important mediators of absorption, distribution, metabolism and excretion of drug substances. This review gives a current status on the transporter(s) mediating the absorption, distribution, metabolism and excretion properties of the anti-epileptic drug substance vigabatrin. For orally administered drugs, like vigabatrin, the absorption from the intestine is a prerequisite for the bioavailability. Therefore, transporter(s) involved in the intestinal absorption of vigabatrin in vitro and in vivo are discussed in detail. Special focus is on the contribution of the proton-coupled amino acid transporter 1 (PAT1) for intestinal vigabatrin absorption. Furthermore, the review gives an overview of the pharmacokinetic parameters of vigabatrin across different species and drug-food and drug-drug interactions involving vigabatrin. The aims were to determine blood-brain barrier penetration and brain extracellular pharmacokinetics for the anticonvulsant vigabatrin (VGB; gamma-vinyl-gamma-aminobutyric acid) in brain extracellular fluid and plasma from severe traumatic brain injury (TBI) patients, and to measure the response of gamma-aminobutyric acid (GABA) concentration in brain extracellular fluid. Severe TBI patients (n = 10) received VGB (0.5 g enterally, every 12 hr). Each patient had a cerebral microdialysis catheter; two patients had a second catheter in a different region of the brain. Plasma samples were collected 0.5 hr before and 2, 4 and 11.5 hr after the first VGB dose. Cerebral microdialysis commenced before the first VGB dose and continued through at least three doses of VGB. Controls were seven severe TBI patients with microdialysis, without VGB. After the first VGB dose, the maximum concentration of VGB (Cmax) was 31.7 (26.9-42.6) umol/L (median and interquartile range for eight patients) in plasma and 2.41 (2.03-5.94) umol/L in brain microdialysates (nine patients, 11 catheters), without significant plasma-brain correlation. After three doses, median Cmax in microdialysates increased to 5.22 (4.24-7.14) umol/L (eight patients, 10 catheters). Microdialysate VGB concentrations were higher close to focal lesions than in distant sites. Microdialysate GABA concentrations increased modestly in some of the patients after VGB administration. Vigabatrin, given enterally to severe TBI patients, crosses the blood-brain barrier into the brain extracellular fluid, where it accumulates with multiple dosing. Pharmacokinetics suggest delayed uptake from the blood. /MILK/ Vigabatrin distributes into milk, probably in small amounts. The aim of the study was to investigate the intestinal transport mechanisms responsible for vigabatrin absorption in rats by developing a population pharmacokinetic (PK) model of vigabatrin oral absorption. The PK model was used to investigate whether vigabatrin absorption was carrier-mediated and if the proton-coupled amino acid transporter 1 (PAT1) was involved in the absorption processes. Vigabatrin (0.3-300 mg/kg) was administered orally or intravenously to Sprague Dawley rats in the absence or presence of PAT1-ligands l-proline, l-tryptophan or sarcosine. The PK profiles of vigabatrin were described by mechanistic non-linear mixed effects modelling, evaluating PAT1-ligands as covariates on the PK parameters with a full covariate modelling approach. The oral absorption of vigabatrin was adequately described by a Michaelis-Menten type saturable absorption. Using a Michaelis constant of 32.8 mM, the model estimated a maximal oral absorption rate (Vmax) of 64.6mmol/min and dose-dependent bioavailability with a maximum of 60.9%. Bioavailability was 58.5-60.8% at 0.3-30 mg/kg doses, but decreased to 46.8% at 300 mg/kg. Changes in oral vigabatrin PK after co-administration with PAT1-ligands was explained by significant increases in the apparent Michaelis constant. Based on the mechanistic model, a high capacity low affinity carrier is proposed to be involved in intestinal vigabatrin absorption. PAT1-ligands increased the Michaelis constant of vigabatrin after oral co-administration indicating that this carrier could be PAT1. For more Absorption, Distribution and Excretion (Complete) data for Vigabatrin (10 total), please visit the HSDB record page. Metabolism / Metabolites Vigabatrin is not metabolized to any significant extent. Vigabatrin is not significantly metabolized ... . Almost no metabolic transformation. Does not induce the hepatic cytochrome P450 system. Route of Elimination: Eliminated primarily through renal excretion as unchanged drugs (80%). Half Life: Neonates, 50 mg/kg = 7.5 ± 2.1 hours (due to reduced renal function); Infants = 5.7 hours; Adults = 7.5 hours; Elderly = 12 - 13 hours Biological Half-Life The terminal half-life of vigabatrin is approximately 5.7 hours for infants (5m - 2y), 6.8 hours for children (3y - 9y), 9.5 hours for adolescents (10y - 16y), and 10.5 h for adults. The terminal half-life of vigabatrin is about 5.7 hours for infants (5 months - 2 years), 9.5 hours for children (10 years - 16 years), and 10.5 hours for adults. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Vigabatrin is a structural analog of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the CNS. Vigabatrin is commercially available as a racemic mixture of 2 enantiomers; the S enantiomer is pharmacologically active and the R enantiomer is inactive. HUMAN STUDIES: Visual field defects, including permanent vision loss, have been reported in infants, children, and adults receiving vigabatrin. Based on clinical studies in adults, bilateral concentric visual field constriction ranging in severity from mild to severe may occur in 30% or more of patients receiving the drug. Severe cases may be characterized by tunnel vision to within 10 degrees of visual fixation, which can lead to disability. In some cases, vigabatrin can also damage the central retina and decrease visual acuity. Coma, unconsciousness, and/or drowsiness were described in the majority of cases of vigabatrin overdose. Other less commonly reported symptoms included vertigo, psychosis, apnea or respiratory depression, bradycardia, agitation, irritability, confusion, headache, hypotension, abnormal behavior, increased seizure activity, status epilepticus, and speech disorder. These symptoms resolved with supportive care. ANIMAL STUDIES: Vigabatrin showed no carcinogenic potential in mouse or rat when given in the diet at doses up to 150 mg/kg/day for 18 months (mouse) or at doses up to 150 mg/kg/day for 2 years (rat). Vigabatrin (300 or 450 mg/kg) was administered by intraperitoneal injection to a mutant mouse strain on a single day during organogenesis (day 7, 8, 9, 10, 11, or 12). An increase in malformations (including cleft palate) was observed at both doses. In rats, oral administration of vigabatrin (50, 100, or 150 mg/kg) throughout organogenesis resulted in decreased fetal body weights and increased incidences of fetal anatomic variations. Oral administration of vigabatrin (50, 100, 150 mg/kg) to rats from the latter part of pregnancy through weaning produced long-term neurohistopathological (hippocampal vacuolation) and neurobehavioral (convulsions) abnormalities in the offspring. Administration of vigabatrin (oral doses of 50 to 200 mg/kg) to pregnant rabbits throughout the period of organogenesis was associated with an increased incidence of malformations (cleft palate) and embryo-fetal death; these findings were observed in two separate studies. No adverse effects on male or female fertility were observed in rats at oral doses up to 150 mg/kg/day. Oral administration of vigabatrin (5, 15, or 50 mg/kg) to young rats during the neonatal and juvenile periods of development (postnatal days 4-65) produced neurobehavioral (convulsions, neuromotor impairment, learning deficits) and neurohistopathological (brain vacuolation, decreased myelination, and retinal dysplasia) abnormalities in treated animals. The early postnatal period in rats is generally thought to correspond to late pregnancy in humans in terms of brain development. Vigabatrin was negative in in vitro (Ames, CHO/HGPRT mammalian cell forward gene mutation, chromosomal aberration in rat lymphocytes) and in in vivo (mouse bone marrow micronucleus) assays. Vigabatrin increases brain concentrations of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter in the CNS, by irreversibly inhibiting enzymes that catabolize GABA (gamma-aminobutyric acid transaminase, GABA-T). Duration of action is determined by rate of GABA-T re-synthesis. Vigabatrin may also work by suppressing repetitive neuronal firing through inhibition of voltage-sensitive sodium channels. Although administered as a racemic mixture, only the S(+) enantiomer is pharmacologically active. Hepatotoxicity In controlled clinical trials, addition of vigabatrin to standard anticonvulsant therapy was reported to cause an immediate and marked decrease in serum enzyme levels that could be reproduced by simply mixing vigabatrin with plasma. In some instances, markedly raised serum ALT levels were found to rapidly fall into the normal range with treatment. Vigabatrin inhibits GABA transaminase and is thus suspected of also being an inhibitor of alanine and aspartate aminotransferase, accounting for its unusual effects on liver associated enzymes. In prelicensure clinical trials, there were no reports of serum enzyme elevations during treatment and no instances of clinically apparent liver injury. After its general availability, however, there have been isolated case reports of severe liver injury and hepatitis associated with vigabatrin use. The onset of injury was 3 to 10 months after starting vigabatrin and was largely hepatocellular. One case resulted in rapid death from liver failure and a second worsened despite stopping and ultimately required a course of immunosuppression with prednisone and azathioprine (Case 1). Thus, clinically apparent liver injury from vigabatrin may occur and can be severe, but is rare. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal doses of vigabatrin up to 2000 mg daily produce low levels in milk. Vigabatrin is approved for use in infants in one month and older and amounts in milk are far less than the approved infant dosage. Vigabatrin would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Vigabatrin does not bind to plasma proteins. Toxicity Data LD50, oral, rat: 3000 mg/kg Interactions Sabril may moderately increase the Cmax of clonazepam resulting in an increase of clonazepam-associated adverse reactions. Based on population pharmacokinetic modeling, concurrent administration of vigabatrin and rufinamide appears to be associated with a slight to moderate decrease in mean steady-state plasma concentrations of rufinamide (ranging from a decrease of approximately 14-15% in adults to a decrease of approximately 30% in children). Although the clinical importance of this potential interaction remains to be established, some clinicians recommend careful patient monitoring when either anticonvulsant is initiated or discontinued; rufinamide dosage adjustment should be considered if clinically necessary. In controlled clinical studies, concomitant administration of phenytoin and vigabatrin resulted in moderate reductions (averaging 16-20%) in total plasma phenytoin concentrations, probably due to induction of CYP2C9. In a pharmacokinetic study evaluating a possible interaction between vigabatrin and phenytoin, mean plasma phenytoin concentrations fell by 23% during the fifth week of concurrent administration. Such reductions may be of little clinical importance, and phenytoin dosage adjustments are not routinely required; however, phenytoin dosage adjustment should be considered if clinically indicated. In a study in healthy individuals, concomitant administration of vigabatrin (1.5 g twice daily) with clonazepam (0.5 mg) did not affect plasma concentrations of vigabatrin; however, mean peak plasma clonazepam concentrations increased by 30% and mean time to peak clonazepam concentrations decreased by 45%, which may increase the risk of clonazepam-associated adverse effects. In another study in healthy individuals, vigabatrin did not appear to potentiate the CNS effects of clonazepam during concurrent administration. For more Interactions (Complete) data for Vigabatrin (7 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anticonvulsants; Enzyme Inhibitors; GABA Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Vigabatrin is included in the database. Sabril is indicated as adjunctive therapy for adults and pediatric patients 10 years of age and older with refractory complex partial seizures who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss. Sabril is not indicated as a first line agent for complex partial seizures. /Included in US product label/ Sabril is indicated as monotherapy for pediatric patients with infantile spasms 1 month to 2 years of age for whom the potential benefits outweigh the potential risk of vision loss. /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: PERMANENT VISION LOSS. Sabril can cause permanent bilateral concentric visual field constriction, including tunnel vision that can result in disability. In some cases, Sabril also can damage the central retina and may decrease visual acuity. The onset of vision loss from Sabril is unpredictable, and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years. Symptoms of vision loss from Sabril are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function. The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss. Vision assessment is recommended at baseline (no later than 4 weeks after starting Sabril), at least every 3 months during therapy, and about 3 to 6 months after the discontinuation of therapy. Once detected, vision loss due to Sabril is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss. Consider drug discontinuation, balancing benefit and risk, if visual loss is documented. Risk of new or worsening vision loss continues as long as Sabril is used. It is possible that vision loss can worsen despite discontinuation of Sabril. Because of the risk of vision loss, Sabril should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2-4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for Sabril should be periodically reassessed. Sabril should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks. Sabril should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks. Use the lowest dosage and shortest exposure to Sabril consistent with clinical objectives. Because of the risk of permanent vision loss, Sabril is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program Visual field defects, including permanent vision loss, have been reported in infants, children, and adults receiving vigabatrin. Based on clinical studies in adults, bilateral concentric visual field constriction ranging in severity from mild to severe may occur in 30% or more of patients receiving the drug. Severe cases may be characterized by tunnel vision to within 10 degrees of visual fixation, which can lead to disability. In some cases, vigabatrin can also damage the central retina and decrease visual acuity. Because vision assessment may be difficult in infants and children, the frequency and extent of vision loss is poorly characterized in such patients; therefore, the understanding of the risk is mainly based on adult experience with the drug. The possibility that vigabatrin-induced vision loss may be more common, more severe, or have more functional consequences in infants and children than in adults cannot be excluded. The onset and progression of vision loss with vigabatrin are unpredictable and can occur within weeks of beginning treatment or sooner or at any time after starting therapy, even after months or years. In addition, vision loss may develop or worsen precipitously between vision assessments. Symptoms of vigabatrin-associated vision loss are unlikely to be recognized by patients or caregivers before the impairment is severe. Vision loss of milder severity that is often unrecognized by the patient or caregiver can still adversely affect function. Once detected, vigabatrin-induced visual field defects are irreversible and will not improve even after the drug is discontinued. In addition, it is possible that further impairment of vision may occur following drug discontinuance. Risk of vision loss increases with increasing dosages and cumulative exposure to vigabatrin; however, no dosage or exposure to the drug is known to be free of the risk of vision loss. Some studies have suggested that smoking, age, and male gender are possible risk factors for developing visual field defects. In patients with infantile spasms, vigabatrin therapy should be withdrawn if a substantial clinical benefit is not observed within 2-4 weeks of initiating the drug. If, in the clinical judgment of the prescribing clinician, evidence of treatment failure becomes obvious earlier than 2-4 weeks, vigabatrin treatment should be discontinued at that time. For more Drug Warnings (Complete) data for Vigabatrin (25 total), please visit the HSDB record page. Pharmacodynamics Vigabatrin is an antiepileptic agent chemically unrelated to other anticonvulsants. Vigabatrin prevents the metabolism of GABA by irreversibly inhibiting GABA transaminase (GABA-T). As vigabatrin is an irreversible inhibitor of gamma-aminobutyric acid transaminase (GABA-T), its duration of effect is thought to be dependent on the rate of GABA-T re-synthesis rather than on the rate of drug elimination. |
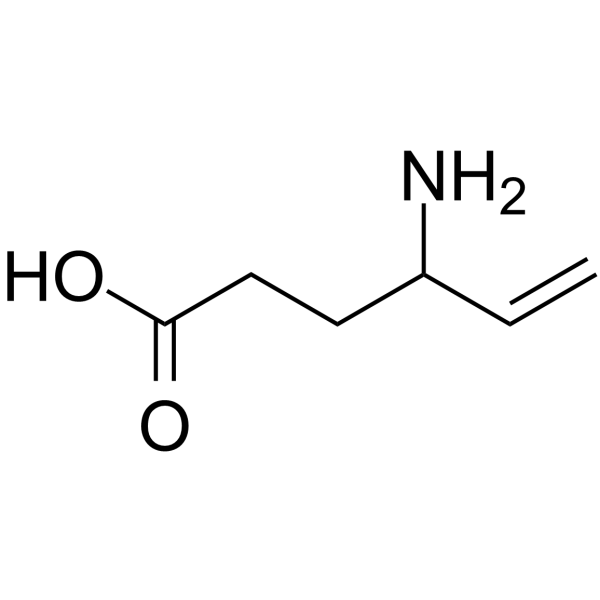
| 分子式 |
C6H11NO2
|
|---|---|
| 分子量 |
129.15704
|
| 精确质量 |
129.078
|
| CAS号 |
68506-86-5
|
| 相关CAS号 |
Vigabatrin hydrochloride;1391054-02-6
|
| PubChem CID |
5665
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
277.7±28.0 °C at 760 mmHg
|
| 熔点 |
171-176C
209 °C |
| 闪点 |
121.7±24.0 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.483
|
| LogP |
-0.1
|
| tPSA |
63.32
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
112
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
PJDFLNIOAUIZSL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)
|
| 化学名 |
4-aminohex-5-enoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~50 mg/mL (~387.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (774.23 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.7423 mL | 38.7117 mL | 77.4234 mL | |
| 5 mM | 1.5485 mL | 7.7423 mL | 15.4847 mL | |
| 10 mM | 0.7742 mL | 3.8712 mL | 7.7423 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。