| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
| 靶点 |
Rat 5-HT1A Receptor ( Ki = 3.4 nM ); human 5-HT1A Receptor ( Ki = 2.5 nM ); Rat D2 Receptor ( Ki = 4.8 nM ); Rat 5-HT2A ( Ki = 0.42 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:齐拉西酮对人类 5-HT 受体和人类多巴胺 D(2) 受体具有高亲和力。 Ziprasidone 是一种 5-HT(1A) 受体激动剂,也是 5-HT(2A)、5-HT(2C) 和 5-HT(1B/1D) 受体拮抗剂。齐拉西酮抑制神经元对 5-HT 和去甲肾上腺素的摄取,与抗抑郁药丙咪嗪相当。在稳定转染的 HEK-293 细胞中,齐拉西酮以电压和浓度依赖性方式阻断野生型 hERG 电流,IC(50) 为 120nM。齐拉西酮显示出在去极化电压(-20或+30mV)期间估计的或通过尾部包络测试(+30mV)评估的hERG电流的最小强直阻滞。齐拉西酮显着增加 hERG 电流失活慢分量的时间常数(-50mV)。
|
| 体内研究 (In Vivo) |
齐拉西酮对野生型 hERG 电流的阻断作用较弱,在非洲爪蟾卵母细胞中的 IC(50) 为 2.8 mM。齐拉西酮抑制奥氮平引起的食物摄入量显着增加,表明它对药物引起的大鼠食物摄入量增加具有内在的保护机制。齐拉西酮导致大鼠海马齿状回 (DG)、CA1 和 CA3 区域的 NGF 和 ChAT 免疫反应性显着增加。齐拉西酮剂量依赖性地减慢麻醉大鼠中缝单位活性(ED50 = 300 mg/kg iv),非典型抗精神病药氯氮平(ED50 = 250 mg/kg iv)和奥氮平(ED50 = 1000 mg/kg iv)也是如此。
|
| 细胞实验 |
细胞系:HEK-293 细胞
浓度:0-500 nM 孵育时间:150 秒 结果:以电压和浓度依赖性方式阻断野生型 hERG 电流(IC50 = 120 nm) 。 |
| 动物实验 |
Eight-week-old female Sprague-Dawley rats weighing 200 to 250 g
20 mg/kg Oral gavage; 20 mg/kg; once daily; 7 weeks |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In the absence of food, ziprasidone's oral bioavailability is 60%, and absorption may reach 100% if ziprasidone is taken with a meal containing at least 500 kcal. The difference in bioavailability has little to do with the fat content of the food and appears to be related to the bulk of the meal since more absorption occurs the longer ziprasidone remains in the stomach. Ziprasidone is extensively metabolized after oral administration with only a small amount excreted in the urine (<1%) or feces (<4%) as unchanged drug. The mean apparent volume of distribution of Ziprasidone is 1.5 L/kg. The mean apparent systemic clearance is 7.5 mL/min/kg. Ziprasidone is well absorbed after oral administration, reaching peak plasma concentrations in 6 to 8 hours. The absolute bioavailability of a 20 mg dose under fed conditions is approximately 60%. The absorption of ziprasidone is increased up to two-fold in the presence of food. The bioavailability of ziprasidone administered intramuscularly is 100%. After intramuscular administration of single doses, peak serum concentrations typically occur at approximately 60 minutes post-dose or earlier ... Steady-state concentrations are achieved within one to three days of dosing. The mean apparent systemic clearance is 7.5 mL/min/kg. Ziprasidone has a mean apparent volume of distribution of 1.5 L/kg. It is greater than 99% bound to plasma proteins, binding primarily to albumin and alpha1-acid glycoprotein. For more Absorption, Distribution and Excretion (Complete) data for Ziprasidone (11 total), please visit the HSDB record page. Metabolism / Metabolites Ziprasidone is heavily metabolized in the liver with less than 5% of the drug excreted unchanged in the urine. The primary reductive pathway is catalyzed by aldehyde oxidase, while 2 other less prominent oxidative pathways are catalyzed by CYP3A4. Ziprasidone is unlikely to interact with other medications metabolized by CYP3A4 since only 1/3 of the antipsychotic is metabolized by the CYP3A4 system. There are 12 identified ziprasidone metabolites (abbreviations italicized): Ziprasidone sulfoxide, ziprasidone sulfone, (6-chloro-2-oxo-2,3-dihydro-1H-indol-5-yl)acetic acid (_OX-COOH_), OX-COOH glucuronide, 3-(piperazine-1-yl)-1,2-benzisothiazole (_BITP_), BITP sulfoxide, BITP sulfone, BITP sulfone lactam, S-Methyl-dihydro-ziprasidone, S-Methyl-dihydro-ziprasidone-sulfoxide, 6-chloro-5-(2-piperazin-1-yl-ethyl)-1,3-dihydro-indol-2-one (_OX-P_), and dihydro-ziprasidone-sulfone. As suggested by the quantity of metabolites, ziprasidone is metabolized through several different pathways. Ziprasidone is sequentially oxidized to ziprasidone sulfoxide and ziprasidone sulfone, and oxidative N-dealkylation of ziprasidone produces OX-COOH and BITP. OX-COOH undergoes phase II metabolism to yield a glucuronidated metabolite while BITP is sequentially oxidized into BITP sulfoxide, BITP sulfone, then BITP sulfone lactam. Ziprasidone can also undergo reductive cleavage and methylation to produce S-Methyl-dihydro-ziprasidone and then further oxidation to produce S-Methyl-dihydro-ziprasidone-sulfoxide. Finally dearylation of ziprasidone produces OX-P, and the process of hydration and oxidation transforms the parent drug into dihydro-ziprasidone-sulfone. Although CYP3A4 and aldehyde oxidase are the primary enzymes involved in ziprasidone metabolism, the pathways associated with each enzyme have not been specified. Ziprasidone is extensively metabolized in the liver principally via reduction by aldehyde oxidase with minimal excretion of unchanged drug in urine or feces. About one-third of ziprasidone's metabolic clearance is mediated by the cytochrome P-450 (CYP) 3A4 isoenzyme. Ziprasidone is primarily cleared via three metabolic routes to yield four major circulating metabolites, benzisothiazole (BITP) sulphoxide, BITP-sulphone, ziprasidone sulphoxide, and S-methyl-dihydroziprasidone. In vitro studies using human liver subcellular fractions indicate that S-methyl-dihydroziprasidone is generated in two steps. The data indicate that the reduction reaction is mediated by aldehyde oxidase and the subsequent methylation is mediated by thiol methyltransferase. In vitro studies using human liver microsomes and recombinant enzymes indicate that CYP3A4 is the major CYP contributing to the oxidative metabolism of ziprasidone. CYP1A2 may contribute to a much lesser extent. Based on in vivo abundance of excretory metabolites, less than one-third of ziprasidone metabolic clearance is mediated by cytochrome P450 catalyzed oxidation and approximately two-thirds via reduction by aldehyde oxidase. There are no known clinically relevant inhibitors or inducers of aldehyde oxidase. The metabolism and excretion of ziprasidone (5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-+++chloroindolin-2-one hydrochloride hydrate) were studied in Long Evans rats after oral administration of a single dose of a mixture of 14C- and 3H-labeled ziprasidone. ... Ziprasidone was extensively metabolized in rats, and only a small amount of ziprasidone was excreted as unchanged drug. Twelve metabolites were identified ... The structures of eight metabolites were unambiguously confirmed by coelution on HPLC with synthetic standards, and four additional metabolites were partially identified. There was a gender-related difference in the excretion of urinary metabolites in Long Evans rats. The major route of metabolism in male rats involved N-dealkylation. In female rats the major metabolites were due to oxidation at the benzisothiazole ring. Based on the structures of these metabolites, four major and two minor routes of metabolism of ziprasidone were identified. The major routes included 1) N-dealkylation of the ethyl side chain attached to the piperazinyl nitrogen, 2) oxidation at the sulfur, resulting in the formation of sulfoxide and sulfone, 3) oxidation on the benzisothiazole moiety (other than sulfur), and 4) hydration of the C==N bond and subsequent oxidation at the sulfur of the benzisothiazole moiety. The minor routes involved N-oxidation on the piperazine ring and hydrolysis of the oxindole moiety. Ziprasidone has known human metabolites that include 6-Chloro-5-ethyl-1,3-dihydroindol-2-one, 3-(1-Piperazinyl)-1,2-benzisothiazole, and Ziprasidone Sulfoxide. Biological Half-Life The half life of ziprasidone is 6-7 hours. Elimination of ziprasidone is mainly via hepatic metabolism with a mean terminal half-life of about 7 hours within the proposed clinical dose range. The mean t(1/2), z in the young men, young women, elderly men and elderly women were 3.1, 4.1, 5.7 and 5.3 hr, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Ziprasidone is indicated for the treatment of schizophrenia. Ziprasidone is indicated as monotherapy for the acute treatment of manic or mixed episodes associated with bipolar I disorder. Ziprasidone is indicated as an adjunct to lithium or valproate for the maintenance treatment of bipolar I disorder. HUMAN EXPOSURE AND TOXICITY: In the patient taking the largest confirmed amount, 3240 mg, the only symptoms reported were minimal sedation, slurring of speech, and transitory hypertension (200/95). In post-marketing use, adverse events reported in association with ziprasidone overdose generally included extrapyramidal symptoms, somnolence, tremor, and anxiety. Previously reported pediatric ziprasidone overdoses describe a syndrome of sedation, tachycardia, hypotonia, and coma. In pediatric ziprasidone overdose, QTc prolongation and hypotension have also been illustrated, but seizures have not been reported. An interesting case of ziprasidone intoxication involving the development of pinpoint pupils unresponsive to naloxone has been reported. This phenomenon has been reported before with overdose of olanzapine, a similar atypical antipsychotic. The mechanism of miosis associated with overdose of atypical antipsychotics is unclear but is likely related to interference with central innervation of the pupil. Geriatric patients with dementia-related psychosis treated with atypical antipsychotic drugs appear to be at an increased risk of death compared with that among patients receiving placebo. In one study, oral ziprasidone prolonged the QT interval on ECG by a mean of 9-14 msec more than that observed in patients receiving risperidone, olanzapine, quetiapine, or haloperidol, but approximately 14 msec less than that observed in patients receiving thioridazine. A ziprasidone overdose with quantitative serum levels of a pediatric patient in coma and with pinpoint pupils illustrating that ingestion of just 1 pill may result to profound mental status and respiratory depression in a child. Positive results were obtained in an in vitro chromosomal aberration assay in human lymphocytes. Psychiatric patients treated with atypical antipsychotic medications should be closely monitored for rhabdomyolysis during correction of hyponatremia, thus permitting prompt therapy to limit its complications. ANIMAL STUDIES: Lifetime carcinogenicity studies were conducted with ziprasidone in rats and mice. Ziprasidone was administered for 24 months in the diet at doses of 2, 6, or 12 mg/kg/day to rats, and 50, 100, or 200 mg/kg/day to mice (0.1 to 0.6 and 1 to 5 times the maximum recommended human dose [MRHD] of 200 mg/day on a sq m basis, respectively). In the rat study, there was no evidence of an increased incidence of tumors compared to controls. In male mice, there was no increase in incidence of tumors relative to controls. In female mice, there were dose-related increases in the incidences of pituitary gland adenoma and carcinoma, and mammary gland adenocarcinoma at all doses tested (50 to 200 mg/kg/day or 1 to 5 times the MRHD on an mg/sq m basis). Proliferative changes in the pituitary and mammary glands of rodents have been observed following chronic administration of other antipsychotic agents and are considered to be prolactin-mediated. Increases in serum prolactin were observed in a 1-month dietary study in female, but not male, mice at 100 and 200 mg/kg/day (or 2.5 and 5 times the MRHD on an mg/sq m basis). Ziprasidone had no effect on serum prolactin in rats in a 5-week dietary study at the doses that were used in the carcinogenicity study. Ziprasidone failed to induce significant weight gain during weeks 1-3, however, significant weight gain was observed on day 28 at 2.5 mg/kg (p < 0.05). Ziprasidone had no effect on food intake at any time point. A significant reduction in water intake (p < 0.05) was observed during the first week of treatment with 2.5 mg/kg ziprasidone. Ziprasidone had no effect on intra-abdominal fat weight, wet or dry uterine weight or plasma prolactin levels. All ziprasidone treated animals displayed a normal four-day estrous cycle. In rats, embryofetal toxicity (decreased fetal weights, delayed skeletal ossification) was observed following administration of 10 to 160 mg/kg/day during organogenesis or throughout gestation, but there was no evidence of teratogenicity. Doses of 40 and 160 mg/kg/day were associated with maternal toxicity. Ziprasidone was tested in the Ames bacterial mutation assay, the in vitro mammalian cell gene mutation mouse lymphoma assay, and the in vivo chromosomal aberration assay in mouse bone marrow. There was a reproducible mutagenic response in the Ames assay in one strain of S. typhimurium in the absence of metabolic activation. Positive results were obtained in the in vitro mammalian cell gene mutation assay. Interactions In vivo studies have revealed an approximately 35% decrease in ziprasidone AUC by concomitantly administered carbamazepine, an approximately 35-40% increase in ziprasidone AUC by concomitantly administered ketoconazole, but no effect on ziprasidone's pharmacokinetics by cimetidine or antacid. Pharmacokinetic/pharmacodynamic studies between ziprasidone and other drugs that prolong the QT interval have not been performed. An additive effect of ziprasidone and other drugs that prolong the QT interval cannot be excluded. Therefore, ziprasidone should not be given with dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, pimozide, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol or tacrolimus. Ziprasidone is also contraindicated with drugs that have demonstrated QT prolongation as one of their pharmacodynamic effects and have this effect described in the full prescribing information as a contraindication or a boxed or bolded warning. Because of its potential for inducing hypotension, ziprasidone may enhance the effects of certain antihypertensive agents. Ziprasidone may antagonize the effects of levodopa and dopamine agonists. For more Interactions (Complete) data for Ziprasidone (8 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antipsychotic Agents; Dopamine Antagonists; Serotonin Antagonists Ziprasidone is indicated for the treatment of schizophrenia. The efficacy of oral ziprasidone was established in four short-term (4- and 6-week) controlled trials of adult schizophrenic inpatients and in one maintenance trial of stable adult schizophrenic inpatients. /Included in US product label/ Ziprasidone is indicated as monotherapy for the acute treatment of manic or mixed episodes associated with bipolar I disorder. Efficacy was established in two 3-week monotherapy studies in adult patients /Included in US product label/ Ziprasidone is indicated as an adjunct to lithium or valproate for the maintenance treatment of bipolar I disorder. Efficacy was established in a maintenance trial in adult patients. The efficacy of ziprasidone as monotherapy for the maintenance treatment of bipolar I disorder has not been systematically evaluated in controlled clinical trials /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Ziprasidone is not approved for the treatment of patients with Dementia-Related Psychosis. Contraindications /include/ known history of QT prolongation (including congenital long QT syndrome), recent acute myocardial infarction, or uncompensated heart failure. Concomitant therapy with other drugs that prolong the QT interval. Known hypersensitivity to ziprasidone. Geriatric patients with dementia-related psychosis treated with atypical antipsychotic drugs appear to be at an increased risk of death compared with that among patients receiving placebo. Analyses of seventeen placebo-controlled trials (average duration of 10 weeks) revealed an approximate 1.6 - to 1.7-fold increase in mortality among geriatric patients receiving atypical antipsychotic drugs (ie, aripiprazole, olanzapine, quetiapine, risperidone) compared with that in patients receiving placebo. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5% compared with a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. The manufacturer states that ziprasidone is not approved for the treatment of patients with dementia-related psychosis. Prolongation of the QT interval can result in an occurrence of ventricular arrhythmias (eg, torsades de pointes) and/or sudden death. In one study, oral ziprasidone prolonged the QT interval on ECG by a mean of 9-14 msec more than that observed in patients receiving risperidone, olanzapine, quetiapine, or haloperidol, but approximately 14 msec less than that observed in patients receiving thioridazine. ... Patients at particular risk of torsades de pointes and/or sudden death include those with bradycardia, hypokalemia, or hypomagnesemia, those receiving concomitant therapy with other drugs that prolong the QTC interval, and those with congenital prolongation of QTC interval. The manufacturer states that ziprasidone should be avoided in patients with congenital prolongation of the QT interval or a history of cardiac arrhythmias and in those receiving concomitant therapy with other drugs that prolong the QTC interval. For more Drug Warnings (Complete) data for Ziprasidone (28 total), please visit the HSDB record page. Pharmacodynamics Ziprasidone is classified as a "second generation" or "atypical" antipsychotic and is a dopamine and 5HT2A receptor antagonist with a unique receptor binding profile. As previously mentioned, ziprasidone has a very high 5-HT2A/D2 affinity ratio, binds to multiple serotonin receptors in addition to 5-HT2A, and blocks monoamine transporters which prevents 5HT and NE reuptake. On the other hand, ziprasidone has a low affinity for muscarinic cholinergic M1, histamine H1, and alpha1-adrenergic receptors. |
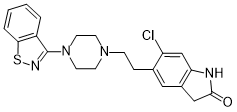
| 分子式 |
C21H21CLN4OS
|
|---|---|
| 分子量 |
412.94
|
| 精确质量 |
412.11
|
| 元素分析 |
C, 61.08; H, 5.13; Cl, 8.58; N, 13.57; O, 3.87; S, 7.76
|
| CAS号 |
146939-27-7
|
| 相关CAS号 |
Ziprasidone-d8; 1126745-58-1; Ziprasidone hydrochloride monohydrate; 138982-67-9; Ziprasidone hydrochloride; 122883-93-6; Ziprasidone mesylate trihydrate; 199191-69-0; Ziprasidone mesylate; 185021-64-1
|
| PubChem CID |
60854
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
554.8±50.0 °C at 760 mmHg
|
| 熔点 |
213-215°C
|
| 闪点 |
289.3±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.681
|
| LogP |
4
|
| tPSA |
76.71
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
573
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C2C(C([H])([H])C(N2[H])=O)=C([H])C=1C([H])([H])C([H])([H])N1C([H])([H])C([H])([H])N(C2C3=C([H])C([H])=C([H])C([H])=C3SN=2)C([H])([H])C1([H])[H]
|
| InChi Key |
MVWVFYHBGMAFLY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27)
|
| 化学名 |
5-[2-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydroindol-2-one
|
| 别名 |
CP 88059; CP88059; CP-88,059-01; CP88059 hydrochloride; CP-88059; CP-88,059; Ziprasidone HCl; Geodon; Zeldox; Zipwell
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~13.5 mg/mL (~32.7 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.35 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 13.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.35 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 13.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.35 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4217 mL | 12.1083 mL | 24.2166 mL | |
| 5 mM | 0.4843 mL | 2.4217 mL | 4.8433 mL | |
| 10 mM | 0.2422 mL | 1.2108 mL | 2.4217 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
An Observational Drug Utilization Study of Asenapine in the United Kingdom (P08308)
CTID: NCT01498770
Phase: Status: Completed
Date: 2022-02-04