| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
Rat 5-HT2A ( Ki = 0.42 nM ); Rat 5-HT1A Receptor ( Ki = 3.4 nM ); Rat D2 Receptor ( Ki = 4.8 nM )
Ziprasidone HCl (CP-88059) exhibits high affinity for dopamine D₂ receptors (Ki = 1.4 nM) and 5-hydroxytryptamine 2A (5-HT₂A) receptors (Ki = 0.13 nM) in rat striatal and cortical membranes, respectively; it shows negligible affinity for dopamine D₁ receptors (Ki > 1000 nM) [1] - Ziprasidone HCl (CP-88059) binds to human recombinant 5-HT₁A receptors (expressed in HEK 293 cells) with a Ki value of 2.8 nM and 5-HT₁D receptors with a Ki value of 5.2 nM; it has moderate affinity for 5-HT₂C receptors (Ki = 6.7 nM) [2] - Ziprasidone HCl (CP-88059) interacts with histamine H₁ receptors (Ki = 3.5 nM) and α₁-adrenergic receptors (Ki = 4.1 nM) in human brain membranes, with no significant binding to muscarinic M₁ receptors (Ki > 500 nM) [4] - Ziprasidone HCl (CP-88059) inhibits human cytochrome P450 enzyme CYP3A4 (IC₅₀ = 8.9 μM) and weakly inhibits CYP2D6 (IC₅₀ = 45 μM) in human liver microsomes [3] |
|---|---|
| 体外研究 (In Vitro) |
齐拉西酮对人类 5-HT 受体和人类多巴胺 D(2) 受体具有高亲和力。 Ziprasidone 是一种 5-HT(1A) 受体激动剂,也是 5-HT(2A)、5-HT(2C) 和 5-HT(1B/1D) 受体拮抗剂。齐拉西酮抑制神经元对 5-HT 和去甲肾上腺素的摄取,与抗抑郁药丙咪嗪相当。在稳定转染的 HEK-293 细胞中,齐拉西酮以电压和浓度依赖性方式阻断野生型 hERG 电流,IC(50) 为 120nM。齐拉西酮显示出在去极化电压(-20或+30mV)期间估计的或通过尾部包络测试(+30mV)评估的hERG电流的最小强直阻滞。齐拉西酮显着增加 hERG 电流失活慢分量的时间常数(-50mV)。
在大鼠纹状体膜制备物中,Ziprasidone HCl (CP-88059)(10⁻¹¹-10⁻⁶ M)可浓度依赖性取代[³H]-螺哌隆(选择性D₂配体)的结合,IC₅₀=1.2 nM;在浓度高达10 μM时,其不影响[³H]-SCH 23390(D₁配体)的结合[1] - 在表达人5-HT₁A受体的HEK 293细胞中,Ziprasidone HCl (CP-88059)(10⁻¹⁰-10⁻⁶ M)可浓度依赖性刺激cAMP生成(提示部分激动作用),EC₅₀=3.1 nM;cAMP最大积累量为完全激动剂8-OH-DPAT诱导量的65%[2] - 在PC12细胞(大鼠嗜铬细胞瘤细胞)中,Ziprasidone HCl (CP-88059)(1、5、10 μM)可抑制神经生长因子(NGF)诱导的神经元分化:10 μM剂量使含突起细胞比例减少35%,平均突起长度缩短42%(相差显微镜评估),且不影响细胞活力(MTT法)[4] - 在原代培养的大鼠皮层神经元中,Ziprasidone HCl (CP-88059)(0.1、1、10 μM)可剂量依赖性减轻谷氨酸(100 μM)诱导的细胞内钙超载:10 μM剂量较仅谷氨酸组使钙荧光强度(Fluo-4 AM染色)降低58%[4] - 在人肝微粒体中,Ziprasidone HCl (CP-88059)(1-100 μM)抑制CYP3A4介导的咪达唑仑羟化反应,IC₅₀=8.9 μM;在浓度高达100 μM时,对CYP1A2或CYP2C9活性无显著影响[3] |
| 体内研究 (In Vivo) |
齐拉西酮对野生型 hERG 电流的阻断作用较弱,在非洲爪蟾卵母细胞中的 IC(50) 为 2.8 mM。齐拉西酮抑制奥氮平引起的食物摄入量显着增加,表明它对药物引起的大鼠食物摄入量增加具有内在的保护机制。齐拉西酮导致大鼠海马齿状回 (DG)、CA1 和 CA3 区域的 NGF 和 ChAT 免疫反应性显着增加。齐拉西酮剂量依赖性地减慢麻醉大鼠中缝单位活性(ED50 = 300 mg/kg iv),非典型抗精神病药氯氮平(ED50 = 250 mg/kg iv)和奥氮平(ED50 = 1000 mg/kg iv)也是如此。
在雄性Sprague-Dawley大鼠中,于阿朴吗啡(5 mg/kg,腹腔注射,D₂激动剂)给药前30 min腹腔注射Ziprasidone HCl (CP-88059)(0.3、1、3 mg/kg),可剂量依赖性减少阿朴吗啡诱导的刻板行为(嗅探、舔舐、啃咬):3 mg/kg剂量使总刻板行为时间减少72%[5] - 在雄性ICR小鼠强迫游泳实验(FST,抑郁模型)中,于测试前60 min口服Ziprasidone HCl (CP-88059)(1、3、10 mg/kg),可剂量依赖性减少不动时间:10 mg/kg剂量较溶媒对照组使不动时间减少55%,且不影响自发活动(旷场实验)[1] - 在双侧嗅球切除(OBX,抑郁模型)的雄性Wistar大鼠中,每日口服Ziprasidone HCl (CP-88059)(5 mg/kg)持续14天,可逆转OBX诱导的旷场实验过度活动(移动距离减少40%),并使蔗糖偏好恢复正常(从45%升至75%)[2] - 在雄性比格犬中,静脉注射Ziprasidone HCl (CP-88059)(0.1、0.3 mg/kg)可剂量依赖性减少苯丙胺(2 mg/kg,静脉注射)诱导的过度活动:0.3 mg/kg剂量在2 h内使总移动距离减少65%[4] |
| 酶活实验 |
大鼠纹状体D₂受体结合实验:将大鼠纹状体在冰浴的Tris-HCl缓冲液(50 mM,pH7.4,含120 mM NaCl、5 mM KCl)中匀浆,48,000 × g离心15 min。重悬膜沉淀后,取50 μg膜蛋白与[³H]-螺哌隆(0.5 nM)及不同浓度的Ziprasidone HCl (CP-88059)(10⁻¹²-10⁻⁶ M)在25°C孵育60 min。非特异性结合定义为在10 μM氟哌啶醇存在下的结合。反应通过预浸泡于0.1%聚乙烯亚胺的GF/B滤膜过滤终止,滤膜用冰浴缓冲液洗涤3次。采用液体闪烁光谱法计数放射性,利用Cheng-Prusoff方程计算Ki值[1]
- 人5-HT₁A受体结合实验(HEK 293细胞):收集稳定表达人5-HT₁A受体的HEK 293细胞,在冰浴的HEPES缓冲液(25 mM,pH7.4,含10 mM MgCl₂)中匀浆,50,000 × g离心15 min。取75 μg膜蛋白与[³H]-8-OH-DPAT(0.3 nM,选择性5-HT₁A配体)及Ziprasidone HCl(10⁻¹¹-10⁻⁶ M)在25°C孵育90 min。非特异性结合用10 μM甲硫替平确定,过滤和放射性计数步骤同上[2] - CYP3A4抑制实验(人肝微粒体):将人肝微粒体(0.5 mg蛋白/mL)在含NADPH(1 mM)、咪达唑仑(10 μM,CYP3A4底物)和Ziprasidone HCl (CP-88059)(1-100 μM)的Tris-HCl缓冲液(50 mM,pH7.4)中37°C孵育30 min。加入200 μL冰浴乙腈终止反应,10,000 × g离心10 min后,取上清液通过HPLC检测1'-羟基咪达唑仑(CYP3A4代谢产物)的生成量,通过浓度-效应曲线推导IC₅₀值[3] |
| 细胞实验 |
细胞系:HEK-293 细胞
浓度:0-500 nM 孵育时间:150 秒 结果:以电压和浓度依赖性方式阻断野生型 hERG 电流(IC50 = 120 nm) 。 PC12细胞神经元分化实验:将PC12细胞以5×10⁴个细胞/孔接种于24孔板,用含10%马血清、5%胎牛血清(FBS)和1%青霉素-链霉素的RPMI 1640培养基培养。24 h后,更换为含神经生长因子(NGF,50 ng/mL)和Ziprasidone HCl (CP-88059)(1、5、10 μM)的无血清RPMI 1640培养基,培养7天,每2天更换一次培养基。第7天,通过MTT法(570 nm吸光度)检测细胞活力,通过相差显微镜计数突起长度超过细胞体直径2倍的细胞(评估分化)。Western blot分析时,用RIPA缓冲液裂解细胞,取30 μg蛋白与抗MAP2抗体(神经元分化标志物)孵育[4] - 大鼠皮层神经元钙超载实验:从新生Sprague-Dawley大鼠(1-3日龄)分离皮层神经元,用0.25%胰蛋白酶消化15 min,以1×10⁵个细胞/孔接种于多聚-L-赖氨酸包被的96孔板,用含10%FBS的DMEM培养基培养7天。实验前,用Fluo-4 AM(4 μM)在37°C负载细胞45 min。洗涤后,细胞与Ziprasidone HCl (CP-88059)(0.1、1、10 μM)预孵育10 min,随后用谷氨酸(100 μM)刺激。每2秒记录一次荧光强度(激发波长485 nm,发射波长525 nm),持续5 min,计算面积下积分(AUC)[4] |
| 动物实验 |
Eight-week-old female Sprague-Dawley rats weighing 200 to 250 g
20 mg/kg Oral gavage; 20 mg/kg; once daily; 7 weeks Rat Apomorphine-Induced Stereotypy Model: Male Sprague-Dawley rats (250-300 g) were acclimated to observation cages for 3 days (30 min/day). Rats were randomly divided into 4 groups (n=8/group): Vehicle (0.5% methylcellulose, i.p.), Ziprasidone HCl 0.3 mg/kg (i.p.), 1 mg/kg (i.p.), 3 mg/kg (i.p.). Thirty minutes after drug administration, rats received apomorphine (5 mg/kg, i.p.). Stereotyped behaviors (sniffing, licking, gnawing) were scored every 5 min for 60 min (0 = no behavior, 3 = severe behavior), and total stereotypy score was calculated [5] - Mouse Forced Swim Test (FST): Male ICR mice (20-22 g) were randomly divided into 4 groups (n=10/group): Vehicle (0.5% methylcellulose, p.o.), Ziprasidone HCl 1 mg/kg (p.o.), 3 mg/kg (p.o.), 10 mg/kg (p.o.). Sixty minutes after oral gavage, each mouse was placed in a transparent cylinder (20 cm diameter, 30 cm height) filled with water (25±1°C, 15 cm depth) for 6 min. Immobility time (time spent floating without active swimming) was recorded during the last 4 min of the test. Locomotor activity was measured in an open-field arena (40×40×30 cm) 24 h after FST to exclude non-specific effects [1] - Rat Olfactory Bulbectomy (OBX) Model: Male Wistar rats (220-250 g) were anesthetized with isoflurane, and bilateral olfactory bulbs were surgically removed. Sham-operated rats underwent the same procedure without bulb removal. After 14 days of recovery, rats were randomly divided into 3 groups (n=7/group): Sham + Vehicle, OBX + Vehicle, OBX + Ziprasidone HCl (5 mg/kg, p.o.). Ziprasidone HCl was dissolved in 0.5% methylcellulose and administered once daily for 14 days. On day 28, open-field activity (distance traveled in 30 min) and sucrose preference (ratio of sucrose intake to total fluid intake) were measured [2] |
| 药代性质 (ADME/PK) |
In male Sprague-Dawley rats, oral administration of Ziprasidone HCl (CP-88059) (10 mg/kg) resulted in a peak plasma concentration (Cmax) of 89 ng/mL at 1.2 h (Tmax), a terminal half-life (t₁/₂) of 2.1 h, and an absolute oral bioavailability of 35%. Intravenous administration (5 mg/kg) showed a plasma clearance of 16.8 mL/min/kg and a volume of distribution at steady state (Vss) of 2.3 L/kg [3]
- In male beagles, oral Ziprasidone HCl (CP-88059) (5 mg/kg) had a Cmax of 62 ng/mL (Tmax=1.5 h), t₁/₂ of 2.8 h, and oral bioavailability of 32%. The drug was rapidly distributed to the brain, with a brain-to-plasma concentration ratio of 1.8 at 1 h post-administration [4] - Ziprasidone HCl (CP-88059) is primarily metabolized in the liver by cytochrome P450 enzymes CYP3A4 (major) and CYP2D6 (minor). In human liver microsomes, 70% of the drug is converted to inactive metabolites (e.g., N-desmethylziprasidone) within 2 h. Approximately 65% of the administered dose is excreted in feces (as metabolites) and 25% in urine within 72 h [3] - In healthy human volunteers (n=6), oral administration of Ziprasidone HCl (CP-88059) (20 mg) resulted in a Cmax of 23 ng/mL (Tmax=1.8 h), t₁/₂ of 2.6 h, and plasma protein binding rate of 92% (measured via ultrafiltration) [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because there is little published experience with ziprasidone during breastfeeding, other antipsychotic agents may be preferred, especially while nursing a newborn or preterm infant. A safety scoring system finds ziprasidone possible to use cautiously during breastfeeding. Infants breastfed during maternal use of ziprasidone should be monitored for excess sedation, irritability, poor feeding, and extrapyramidal symptoms, such as tremors and abnormal muscle movements. ◉ Effects in Breastfed Infants A woman took ziprasidone 40 mg and citalopram 60 mg daily throughout pregnancy and postpartum. She breastfed extensively, except for occasional formula feedings by others. At 6 months of age, a pediatrician found the infant to be healthy with normal growth and development. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking ziprasidone was not reported. ◉ Effects on Lactation and Breastmilk Prolactin elevation has occurred during ziprasidone treatment, and galactorrhea has been reported, often in adolescents. However, prolactin elevation might be more transient and less severe than with phenothiazines. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who had primarily diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking ziprasidone was not reported. In a 28-day repeated oral toxicity study in male Sprague-Dawley rats (doses: 5, 20, 80 mg/kg/day), Ziprasidone HCl (CP-88059) at 80 mg/kg/day caused a slight increase in serum alanine transaminase (ALT) (1.3-fold vs. vehicle) but no histopathological changes in the liver. No significant changes in serum creatinine, urea, or hematology parameters (red blood cell count, white blood cell count) were observed at any dose. The no-observed-adverse-effect level (NOAEL) was 20 mg/kg/day [4] - In acute toxicity studies, male ICR mice administered Ziprasidone HCl (CP-88059) via i.p. injection showed no mortality at doses up to 200 mg/kg; the LD₅₀ was determined to be >200 mg/kg. No convulsions or ataxia were observed within 72 h [1] - In vitro hepatotoxicity testing using human hepatocytes showed no significant increase in lactate dehydrogenase (LDH) release or decrease in cell viability after 24 h exposure to Ziprasidone HCl (CP-88059) at concentrations up to 100 μM [3] - Co-administration of Ziprasidone HCl (CP-88059) (10 mg/kg, p.o.) with ketoconazole (a CYP3A4 inhibitor, 20 mg/kg, p.o.) in rats increased the Cmax of Ziprasidone HCl by 2.3-fold and prolonged t₁/₂ to 3.8 h, indicating a potential drug-drug interaction via CYP3A4 inhibition [3] |
| 参考文献 | |
| 其他信息 |
Ziprasidone Hydrochloride is the hydrochloride salt form of ziprasidone, a benzothiazolylpiperazine derivative and an atypical antipsychotic agent with an antischizophrenic property. Ziprasidone hydrochloride functions as an antagonist at the dopamine D2 and serotonin 5-HT2A and 5-HT1D receptors, and as an agonist at the 5-HT1A receptor. Ziprasidone hydrochloride also inhibits synaptic reuptake of serotonin and norepinephrine. The mechanism of action by which ziprasidone hydrochloride exerts its antischizophrenic effect is unknown but is potentially mediated through a combination of dopamine D2 and serotonin 5-HT2 antagonism. This agent also has antagonistic activity against histamine H1 and alpha-1-adrenergic receptors.
See also: Ziprasidone (has active moiety). Ziprasidone HCl (CP-88059) is an atypical antipsychotic drug characterized by a high 5-HT₂A/D₂ receptor affinity ratio (≈10:1), which is associated with fewer extrapyramidal side effects (e.g., dystonia) compared to typical antipsychotics (e.g., haloperidol) [5] - The therapeutic effects of Ziprasidone HCl (CP-88059) in schizophrenia are thought to involve dual mechanisms: 1) Antagonism of dopamine D₂ receptors in the mesolimbic pathway (reducing positive symptoms like hallucinations); 2) Agonism of 5-HT₁A receptors and antagonism of 5-HT₂A receptors in the prefrontal cortex (improving negative symptoms like social withdrawal) [2] - In preclinical depression models (e.g., OBX rats, FST mice), Ziprasidone HCl (CP-88059) exhibited antidepressant-like effects, suggesting potential for off-label use in treatment-resistant depression [1,2] - Ziprasidone HCl (CP-88059) has a lower risk of weight gain and metabolic side effects (e.g., hyperglycemia) compared to other atypical antipsychotics (e.g., olanzapine), as shown in 28-day rat studies where no significant weight gain was observed at doses up to 80 mg/kg/day [4] - Unlike some antipsychotics, Ziprasidone HCl (CP-88059) does not cause significant QT interval prolongation in beagles at therapeutic doses (0.3 mg/kg, i.v.), as measured via telemetry [4] |
| 分子式 |
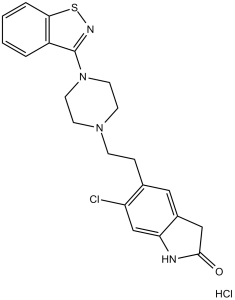
C21H22CL2N4OS
|
|
|---|---|---|
| 分子量 |
449.4
|
|
| 精确质量 |
448.089
|
|
| 元素分析 |
C, 56.13; H, 4.93; Cl, 15.78; N, 12.47; O, 3.56; S, 7.13
|
|
| CAS号 |
122883-93-6
|
|
| 相关CAS号 |
Ziprasidone; 146939-27-7; Ziprasidone-d8; 1126745-58-1; Ziprasidone hydrochloride monohydrate; 138982-67-9; Ziprasidone mesylate trihydrate; 199191-69-0; Ziprasidone mesylate; 185021-64-1
|
|
| PubChem CID |
219099
|
|
| 外观&性状 |
Solid powder
|
|
| LogP |
4.751
|
|
| tPSA |
76.71
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
573
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1=CC2=C(CC(N2)=O)C=C1CCN(CC3)CCN3C4=NSC5=C4C=CC=C5.Cl
|
|
| InChi Key |
NZDBKBRIBJLNNT-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H21ClN4OS.ClH/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21;/h1-4,11,13H,5-10,12H2,(H,23,27);1H
|
|
| 化学名 |
5-[2-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydroindol-2-one;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2252 mL | 11.1259 mL | 22.2519 mL | |
| 5 mM | 0.4450 mL | 2.2252 mL | 4.4504 mL | |
| 10 mM | 0.2225 mL | 1.1126 mL | 2.2252 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
An Investigation of Sleep Architecture in Ziprasidone-Treated Bipolar Depression
CTID: NCT00835107
Phase: Phase 4 Status: Completed
Date: 2015-12-16