| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 100mg | |||
| Other Sizes |
| 靶点 |
Natural product
|
|---|---|
| 体外研究 (In Vitro) |
Arnebin-1对HUVECs增殖的影响[1]
PCNA是核细胞增殖的标志物。为了确定arnebin-1是否促进HUVEC的增殖,通过蛋白质印迹分析测量PCNA水平。在1×10−3µM至10−1µM的浓度范围内,单独使用arnebin-1对PCNA水平没有显著影响(图1B)。然而,在VEGF(1 ng/ml)存在的情况下,arnebin-1以浓度依赖的方式显著增加了PCNA的表达(图1C)。与我们之前的研究结果一致,我们发现arnebin-1对细胞存活率和增殖没有明显影响(因为在测试范围内通过MTT法评估PCNA表达没有变化),但与VEGF具有协同作用,因为它促进了HUVEC的增殖(图5A)。 Arnebin-1激活VEGFR2信号通路[1] 据报道,VEGFR2磷酸化激活了广泛的下游信号底物,这些底物与内皮细胞增殖、迁移和管形成密切相关。为了研究arnebin-1是否激活VEGFR2及其下游信号分子,我们筛选了一些与VEGFR2信号通路相关的基本激酶。如图2所示,arnebin-1以浓度依赖的方式显著增加了VEGF(1 ng/ml)诱导的VEGFR2、FAK、ERK和Src的磷酸化,这表明arnebin-1通过直接靶向VEGFR2并随后激活VEGFR2诱导的下游信号级联来发挥其促血管生成作用。这些结果与我们之前的研究一致,该研究表明,在VEGF存在的情况下,arnebin-1以浓度依赖的方式促进HUVEC的增殖、迁移和管形成。 Arnebin-1以PI3K依赖的方式上调HUVECs中eNOS、VEGF和HIF-1α的表达水平[1] 随后,我们研究了arnebin-1对HUVEC中eNOS和VEGF表达水平的影响。与载体处理的(对照)细胞相比,在1×10−3µM至10−1µM的浓度范围内,arnebin-1以浓度依赖的方式显著增加了HUVEC中eNOS的蛋白质表达(图3A)。此外,与对照组相比,10−2和10−1µM的arnebin-1也显著增加了VEGF蛋白的表达和分泌(图3B和C)。同样,arnebin-1也显著上调了HIF-1α的表达(图3D)。我们进一步研究了arnebin-1在HUVEC中上调eNOS、VEGF和HIF-1α是否是通过其对PI3K通路的影响介导的。在用10-1µM arnebin-1刺激前1小时用2µM LY294002处理,HIF-1α的蛋白表达显著降低(图3E)。同样,用2µM LY294002预处理后,eNOS的蛋白表达以及VEGF蛋白的表达和分泌也显著降低(图3F–H)。 Arnebin-1通过PI3K依赖途径促进HUVEC的增殖、迁移和管形成[1] 在之前的一项研究中,我们证实arnebin-1在VEGF(1 ng/ml)存在下以浓度依赖的方式显著促进HUVEC的增殖、迁移和管形成,在10-1µM时效果最大。在本研究中,我们研究了arnebin-1产生这些作用的机制。如图5所示,与对照组相比,低浓度VEGF(1ng/ml)刺激可增强HUVEC的增殖、迁移和管形成。此外,10−1µM的arnebin-1和VEGF具有协同作用,与单独用VEGF处理的细胞相比,显著增加了这些过程。然而,当HUVEC用PI3K抑制剂LY294002预处理时,arnebin-1和VEGF对细胞增殖、迁移和管形成的协同作用被消除(图5)。如图所示,LY294002预处理减弱了arnebin-1诱导的eNOS、VEGF和HIF-1α表达水平的增加(图3)。总的来说,这些结果表明,arnebin-1通过以PI3K依赖的方式上调eNOS、VEGF和HIF-1α,促进与血管生成相关的内皮细胞增殖、迁移和管形成过程。 |
| 体内研究 (In Vivo) |
Arnebin-1对糖尿病创面HIF-1α、eNOS和VEGF表达的影响[1]
为了研究促进血管新生的机制,在用arnebin-1治疗后,我们测量了HIF-1α及其靶基因、VEGF和eNOS的体内表达水平。Western blot分析显示,与非糖尿病伤口相比,糖尿病伤口中HIF-1α、eNOS和VEGF的蛋白表达水平显著降低(图6)。糖尿病组和赋形剂治疗组的HIF-1α、eNOS和VEGF水平没有显著差异。然而,用arnebin-1治疗后,糖尿病伤口中HIF-1α的表达显著增加(图6A)。与糖尿病组和赋形剂治疗组相比,arnebin-1治疗组的eNOS表达水平更高(图6B)。同样,arnebin-1在第7天显著增加了VEGF的蛋白表达(图6C)。综上所述,这些结果表明,arnebin-1通过上调HIF-1α、eNOS和VEGF的表达水平,促进糖尿病大鼠伤口的新生血管形成。 Arnebin-1对血管新生和糖尿病伤口愈合的影响[1] 在体外实验中,我们证明arnebin-1和低浓度VEGF显著增加了PCNA的表达,而不含VEGF的arnebin-1给药没有达到相同的结果。在体内,糖尿病伤口组织中的VEGF水平仍然较低,与糖尿病组和载体治疗组相比,局部应用arnebin-1软膏可上调PCNA的表达(图7A),这与我们的体外结果一致。为了确定arnebin-1在糖尿病伤口新生血管中的作用,检测了血管生成的生化标志物CD31的表达,以分析arnebin-1的作用。在我们之前的研究中,通过组织学分析,我们证明了用arnebin-1治疗的糖尿病伤口在受伤后第4天和第7天毛细血管密度增加。在本研究中,用抗CD31抗体对内皮细胞进行免疫荧光染色后,非糖尿病大鼠的伤口出现了阳性染色(图7B)。在糖尿病对照动物和载体治疗的糖尿病动物的伤口中,这种染色似乎显著减少。我们发现,在用arnebin-1治疗后的第7天,肉芽形成区域周围的CD31阳性血管数量增加。定量分析结果显示,arnebin-1治疗组的毛细血管密度明显大于糖尿病组(图7C)。此外,蛋白质印迹分析的结果表明,与未用arnebin-1治疗的其他糖尿病组相比,用arnebin1治疗后CD31的蛋白水平显著升高(图7D)。 |
| 细胞实验 |
细胞增殖试验[1]
通过线粒体MTT四氮唑试验检测细胞增殖。将HUVEC以3×103个细胞/孔的速度铺在96孔板上。一夜之间,用或不用LY294002(2µM)预处理HUVEC,然后用补充有载体[二甲亚砜(DMSO)]和Arnebin-1(10-1µM)的测试培养基替换培养基,添加或不添加1ng/ml VEGF。孵育24小时后,根据制造商的说明使用MTT试剂检测活细胞的数量。简而言之,将10µl MTT(5mg/ml)加入100µl培养基中,在37°C下培养4小时。去除上清液后,通过加入DMSO溶解甲赞晶体。使用Biotek Elx-800平板读数器测定培养基的吸光度(570nm)。 细胞迁移试验[1] 如前所述,使用Transwell室进行细胞迁移测定。该装置的底部腔室装有600µl的测试介质。将HUVEC(5×104个细胞/孔)加入上腔,在含有2%FBS的M199培养基中培养。孵育24小时后,去除膜表面上方的未迁移细胞。将迁移细胞用甲醇固定15分钟,然后用0.1%结晶紫染色20分钟。然后用30%冰醋酸冲洗膜。最后,在540nm下检查洗涤溶液以计数HUVEC的数量。 试管形成试验[1] 为了检查促血管生成作用Arnebin-1,我们使用了如前所述的实验性体外Matrigel系统。生长因子降低的Matrigel基底膜基质在4°C的冰上解冻过夜,所有移液管和96孔平底板在使用前均已预冷。在37°C下,每孔用50µl Matrigel涂覆96孔板30分钟。将HUVEC以每孔4×104个细胞的速度接种在100µl的检测培养基中。孵育16小时后,使用倒置显微镜拍摄管状结构。使用ImageJ软件量化总管长。 |
| 动物实验 |
Animals and induction of diabetes [1]
All animal procedures were approved by the Laboratory Animal Center of Sun Yat-sen University. As previously described, male Sprague-Dawley (SD) rats (weighing 250–300 g) were kept in stainless steel cages under pathogen-free conditions. The rats were housed in a controlled environment with a constant temperature of 18–22°C and a 12-h light-dark cycle; the rats were allowed access to food and water ad libitum. The rats were allowed to acclimatize for 4 weeks before the experimental procedures commenced. The rats were fasted for 12 h and were injected intraperitoneally with alloxan monohydrate dissolved in normal saline at a double dose of 100 mg/kg every other day to induce diabetes. Following the administration of alloxan for 3 days, the fasting blood glucose (FBG) levels of the rats were measured using a glucometer. The rats exhibiting FBG levels >16.7 mmol/l were confirmed as diabetic rats for the purposes of our research. The FBG levels were monitored before and after the experiments. The animals were randomly divided into 4 groups (1 non-diabetic and 3 diabetic groups; n=6) as follows: i) the non-diabetic group: rats were administered distilled water for 7 days (non-diabetic group); ii) the first diabetic group: the diabetic animals received distilled water (diabetic group); iii) the second diabetic group: the diabetic animals received the vehicle (ointment without Arnebin-1; DM-vehicle; D + V group); and iv) the third diabetic group: the diabetic animals were treated with arnebin-1 ointment (DM-arnebin-1; D + A group) for 7 days. Preparation of the ointment [1] As described in a previous study of ours, ointment containing siritch (1.5 g), beeswax (5 g) and lard oil (0.15 g) was heated at 70–75°C to become solubilized, and 6.65 mg Arnebin-1 (0.1%) was then added and mixed in. Finally, the mixture was stirred until it cooled to room temperature. This ointment was used as the test compound. Drug administration [1] As stated above, each diabetic rat had 3 wounds on the dorsal surface and the non-diabetic rats had 1 wound. In the D + V group, only wounds on the top of the dorsal side were treated with only the vehicle base (without the test compound). In the diabetic group, only wounds near the tail were treated with distilled water. In the D + A group, only wounds in the middle were treated with Arnebin-1 (0.1% ointment). Thus, in each group of rats, a different wound area was treated. The wounds at the top served as the vehicle controls for the treated wounds. The test compound ointment and the vehicle were applied every other day, in quantities sufficient to cover the wounds with a thin layer. All the treatments were continued until the day of sacrifice. The rats were sacrificed with the use of an intraperitoneal injection of an overdose of barbiturate. Tissue collection [1] The rats were anesthetized with an overdose of pentobarbital (200 mg/kg, injected intraperitoneally) on day 7 post-wounding. The wound and a margin of approximately 5 mm of unwounded skin was excised. These wound tissues were snap-frozen in liquid nitrogen until they were processed for protein isolation. Western blot analysis [1] In order to measure the levels of PCNA, CD31, HIF-1α, VEGF and eNOS in the tissue, wounds treated with Arnebin-1 or the vehicle were harvested on day 7 post-wounding. Following excision, the tissues were homogenized in lysis buffer. The VEGF, eNOS and HIF-1α expression levels were determined by western blot analysis as described above. |
| 参考文献 | |
| 其他信息 |
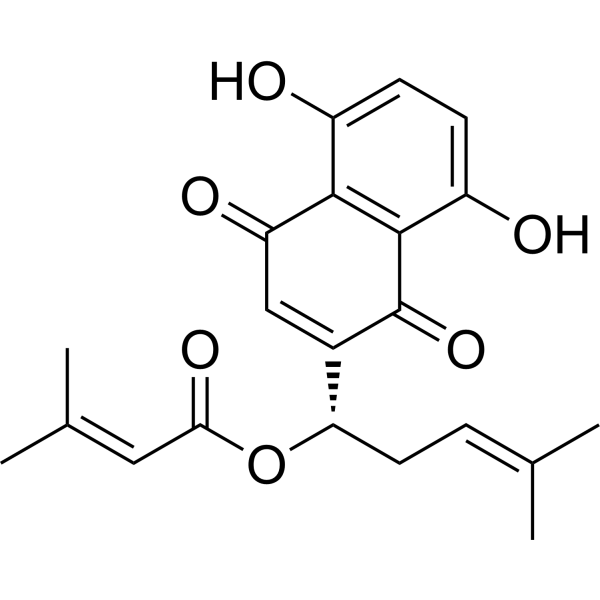
Alkannin beta,beta-dimethylacrylate is a hydroxy-1,4-naphthoquinone.
Alkannin beta,beta-dimethylacrylate has been reported in Arnebia euchroma, Alkanna cappadocica, and Alkanna tinctoria with data available. Arnebin-1, a naphthoquinone derivative, plays a crucial role in the wound healing properties of Zicao (a traditional wound healing herbal medicine). It has been noted that Arnebin-1, in conjunction with vascular endothelial growth factor (VEGF), exerts a synergistic pro-angiogenic effect on human umbilical vein endothelial cells (HUVECs) and accelerates the healing process of diabetic wounds. However, the mechanisms responsible for the pro-angiogenic effect of arnebin‑1 on HUVECs and its healing effect on diabetic wounds have not yet been fully elucidated. In this study, in an aim to elucidate these mechanisms of action of arnebin‑1, we investigated the effects of arnebin‑1 on the VEGF receptor 2 (VEGFR2) and the phosphoinositide 3-kinase (PI3K)‑dependent signaling pathways in HUVECs treated with VEGF by western blot analysis. The pro‑angiogenic effects of arnebin‑1 on HUVECs, including its effects on proliferation and migration, were evaluated by MTT assay, Transwell assay and tube formation assay in vitro. The expression levels of hypoxia-inducible factor (HIF)‑1α, endothelial nitric oxide synthase (eNOS) and VEGF were determined by western blot analysis in the HUVECs and wound tissues obtained from non‑diabetic and diabetic rats. CD31 expression in the rat wounds was evaluated by immunofluorescence staining. We found that the activation of the VEGFR2 signaling pathway induced by VEGF was enhanced by arnebin‑1. Arnebin‑1 promoted endothelial cell proliferation, migration and tube formation through the PI3K‑dependent pathway. Moreover, Arnebin‑1 significantly increased the eNOS, VEGF and HIF‑1α expression levels in the HUVECs and accelerated the healing of diabetic wounds through the PI3K‑dependent signaling pathway. CD31 expression was markedly enhanced in the wounds of diabetic rats treated with arnebin‑1 compared to the wounds of untreated diabetic rats. Therefore, the findings of the present study indicate that arnebin-1 promotes the wound healing process in diabetic rats by eliciting a pro-angiogenic response.[1] In conclusion, based on the outcomes of the present study, and in conjunction with our previous data, we confirmed that arnebin-1 markedly promotes the angiogenesis of HUVECs in vitro and that the topical application of arnebin-1 ointment accelerates the wound healing process in type I diabetic rats by inducing the expression levels of eNOS, VEGF and HIF-1α through the PI3K-dependent signaling pathway. Topical treatment with arnebin-1 ointment may thus be considered a novel therapeutic stratety for diabetic foot ulcers. Clinical tests are warranted to determine whether treatment with arnebin-1 can promote wound healing in patients with diabetes. The exact effects of arnebin-1 on fibroblasts and keratinocytes remain also to be investigated.[1] The present isolation and identification of napthoquinones from roots of Arnebia nobilis Reichb.f. can lead to the discovery of new anti-skin ageing ingredient in colour cosmetics. Four compounds have been isolated and purified by rigorous column chromatography. The compounds are identified as β, β-dimethylacryl alkannin (AN-I), acetoxyisovaleryl alkannin (AAN-II), acetyl alkannin (AN-III) and alkannin (AN-IV) by interpretation of spectroscopic data. This study is the first to report the isolation of Acetoxyisovaleryl alkannin (AAN-II) from A. nobilis. The IC50 values of the compounds, determined in human skin cells (human dermal fibroblasts and human keratinocytes) and mouse embryonic fibroblasts (NIH3T3) varied significantly among the four alkannins. Among the four compounds, β-acetoxyisovaleryl alkannin (AAN-II) significantly inhibited hydrogen peroxide (H2O2)-induced red blood corpuscle haemolysis and cellular senescence in human dermal fibroblasts. Collagen-I, elastin and involucrin syntheses in human dermal fibroblasts or keratinocytes were up regulated by AAN-II. These results support the potential utility of alkannins as novel anti-ageing ingredients.[2] |
| 分子式 |
C21H22O6
|
|---|---|
| 分子量 |
370.3958
|
| 精确质量 |
370.141
|
| CAS号 |
34539-65-6
|
| 相关CAS号 |
(Rac)-Arnebin 1;5162-01-6;β,β-Dimethylacrylshikonin;24502-79-2
|
| PubChem CID |
442720
|
| 外观&性状 |
Brown to reddish brown solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
573.9±50.0 °C at 760 mmHg
|
| 闪点 |
201.1±23.6 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.589
|
| LogP |
5.98
|
| tPSA |
100.9
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
706
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(=CC[C@@H](C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)OC(=O)C=C(C)C)C
|
| InChi Key |
BATBOVZTQBLKIL-KRWDZBQOSA-N
|
| InChi Code |
InChI=1S/C21H22O6/c1-11(2)5-8-17(27-18(25)9-12(3)4)13-10-16(24)19-14(22)6-7-15(23)20(19)21(13)26/h5-7,9-10,17,22-23H,8H2,1-4H3/t17-/m0/s1
|
| 化学名 |
[(1S)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 3-methylbut-2-enoate
|
| 别名 |
34539-65-6; Alkannin beta,beta-dimethylacrylate; b,b-Dimethylacrylalkannin; NCIMech_000202; [(1S)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 3-methylbut-2-enoate; CHEBI:2579; CHEMBL513640; beta,beta-dimethyl-acry-lalkannin;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~23.75 mg/mL (~64.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.75 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6998 mL | 13.4989 mL | 26.9978 mL | |
| 5 mM | 0.5400 mL | 2.6998 mL | 5.3996 mL | |
| 10 mM | 0.2700 mL | 1.3499 mL | 2.6998 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。