| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
Estrogen Receptor/ERR
|
|---|---|
| 体外研究 (In Vitro) |
先前的研究表明,硫酸结合参与了三种常用的癌症药物,他莫昔芬、雷洛昔芬和氟维司坦的代谢。本研究旨在系统鉴定能够硫酸化雷洛昔芬、氟维司琼和他莫昔芬的两种活性代谢产物阿非莫西芬和内莫西芬的人胞质磺基转移酶(SULTs)。使用13种已知的人类SULTs进行的系统分析显示,SULT1A1和SULT1C4是负责阿非莫西芬、内西芬、雷洛昔芬和富韦司琼硫酸化的主要SULTs。测定了这两种人SULTs催化这些药物化合物硫酸化的动力学参数。用HepG2人肝癌细胞和MCF-7乳腺癌症细胞检测了阿非莫昔芬、内昔芬、雷洛昔芬和富司琼在代谢条件下的硫酸化作用。此外,还检查了人的肠、肾、肝和肺细胞溶质,以验证阿非莫西芬/内莫西芬/雷洛昔芬/氟维司琼硫酸化活性的存在。[3]
|
| 体内研究 (In Vivo) |
方法:将年龄至少为18岁、出现中度至重度症状的绝经前妇女随机分为安慰剂组,每天2 mg或4 mg阿非莫昔芬,作为透皮水醇凝胶,持续4个月经周期。主要疗效参数是在从基线到第四个周期的一个周期内,通过视觉模拟量表(VAS)测量的七天最差疼痛评分的平均疼痛强度的变化[4].
结果:4个周期的治疗后,4mg阿非莫昔芬组的平均VAS评分与安慰剂相比有统计学意义的改善(-12.71 mm[95%置信区间,-0.96至-24.47;P=0.034])。与安慰剂组相比,4mg组患者对疼痛的总体评估、医生对疼痛的评估、触诊压痛和4个周期治疗后结节性病变的改善可能性更大(P=0.010[疼痛];P=0.012[压痛];P=0.017[结节性病变])。总体而言,阿非莫昔芬耐受性良好,几乎没有不良事件发生,任何组均未发生与药物相关的SAE。在接受阿非莫昔芬治疗的患者中,月经模式或血浆激素水平没有变化,也没有突破性阴道出血[4]. |
| 酶活实验 |
先前的研究表明,硫酸结合参与了三种常用的癌症药物,他莫昔芬、雷洛昔芬和氟维司坦的代谢。本研究旨在系统地鉴定能够硫酸化雷洛昔芬、氟维司群和三苯氧胺的两种活性代谢产物,即非莫西芬和内莫西芬的人细胞质硫转移酶(SULT)。一项使用13种已知人类SULT的系统分析显示,SULT1A1和SULT1C4是导致非莫西芬、内莫西芬、雷洛昔芬和氟维司群硫酸化的主要SULT。测定了这两种人类SULT催化这些药物化合物硫酸化的动力学参数。用HepG2人肝癌细胞和MCF-7乳腺癌症细胞检测阿非莫昔芬、内昔芬、雷洛昔芬和氟维司琼在代谢条件下的硫酸化作用。此外,还检查了人体肠、肾、肝和肺细胞溶质,以验证是否存在非莫西芬/内莫西芬/雷洛昔芬/氟维司群硫酸化活性[3]。
|
| 细胞实验 |
以阿霉素敏感的K562细胞系和耐药衍生物系KD30和KD225为模型,我们发现获得多药耐药性(MDR)与增强FOXO3a活性和ABCB1(MDR1)表达有关,ABCB1是一种质膜P-糖蛋白,可作为各种抗癌药物的外排泵。此外,阿霉素处理幼稚K562细胞后,ABCB1 mRNA表达的诱导也伴随着FOXO3a活性的增加。对转染的K562、KD30和KD225细胞的分析表明,FOXO3a在蛋白质、mRNA和基因启动子水平上调ABCB1的表达。相反,KD225细胞内源性FOXO3a表达的沉默抑制了这种转运蛋白的表达。启动子分析和染色质免疫沉淀分析表明,FOXO3a对ABCB1表达的调控涉及该转录因子与近端启动子区域的结合。此外,FOXO3a的激活增加了KD30细胞中ABCB1药物的外排潜力,而siRNA沉默FOXO3a显著降低了ABCB1药物外排能力。这些发现共同表明了一种有助于MDR的新机制,涉及FOXO3a作为抗癌药物诱导的细胞毒性应激的传感器。尽管FOXO3a最初可能会触发细胞周期停滞和细胞死亡的程序,以响应阿霉素,但持续的FOXO3a激活通过激活ABCB1表达促进细胞的耐药性和存活。[2]
三苯氧胺广泛应用于治疗雌激素受体(ER)阳性的癌症。最近的研究发现,他莫昔芬及其衍生物4-脱氢氧基-他莫昔芬可以发挥ER非依赖性细胞毒性作用,这促使临床试验开始评估其在ER阴性恶性肿瘤中的应用。例如,三苯氧胺和OHT在不涉及雌激素的恶性周围神经鞘瘤(MPNSTs)中发挥细胞毒性作用。在这项研究中,我们通过研究OHT如何杀死MPNST细胞,深入了解了OHT的ER非依赖性细胞毒性作用。尽管胱天蛋白酶在OHT治疗后被激活,但胱天蛋白酶抑制并不能保护OHT诱导的死亡。相反,OHT诱导的MPNST细胞死亡与自噬诱导有关,并通过基因抑制自噬空泡的形成而减弱。机制研究表明,OHT通过自噬诱导刺激K-Ras降解,这对MPNST细胞的存活至关重要。类似地,我们发现OHT在乳腺、结肠、神经胶质瘤和胰腺癌症细胞中诱导K-Ras降解。我们的研究结果描述了三苯氧胺和OHT在肿瘤细胞中触发自噬死亡的新机制,该机制可能在癌症治疗中更广泛地应用。[5] |
| 动物实验 |
Tamoxifen is a widely used chemotherapeutic agent, which has been associated with prolongation of the QT interval. Other studies have reported that acute exposure to tamoxifen can reduce cardiac K(+) currents. However, in vivo tamoxifen is largely metabolized and most of its activity is attributable to its major metabolite, 4-hydroxytamoxifen (4OH-tamoxifen). Accordingly, in the present study, we performed voltage-clamp experiments to directly investigate the effects of 4OH-tamoxifen on the repolarizing K(+) currents in adult mouse ventricular myocytes in order to determine whether the effects of tamoxifen on repolarization could be ascribed to 4OH-tamoxifen. K(+) currents were recorded before and after acute exposure to 4OH-tamoxifen (0.5, 1 and 10microM). 4OH-tamoxifen reduced the density of the Ca(2+)-independent transient outward (I(to)), the ultrarapid delayed rectifier (I(Kur)) and the inward rectifier (I(K1)) K(+) currents (by up to 43%, 41% and 26%, respectively) but had no significant effect on the steady-state outward K(+) current (I(ss)). Voltage dependence of steady-state inactivation and reactivation time of I(to) and I(Kur) were not affected by 4OH-tamoxifen. Experiments using the pure estrogen receptor antagonist, ICI 182,780 and the inhibitor of gene transcription, actinomycin D, were undertaken to assess the involvement of estrogen receptor. Administered alone these compounds did not affect the density of K(+) currents. Moreover, pretreatment of the cells with ICI 182,780 or actinomycin D did not prevent the inhibitory response to 4OH-tamoxifen. Overall, 4OH-tamoxifen reduced K(+) currents in mouse ventricle and this effect is unrelated to gene transcription and does not involve interaction of the drug with estrogen receptor[6].
Ventricular myocytes were isolated from adult female CD-1 mice (2–3 months) by enzymatic dissociation as previously described. (Fiset et al., 1997, Trépanier-Boulay et al., 2001). All experiments were conducted in accordance with the Canadian Council Animal Care guidelines. The animals were heparinized (1 U/kg; i.p.) 20 min prior to sacrifice, anaesthetized by inhalation of isoflurane and then killed by cervical dislocation. The heart was rapidly excised and retrogradely perfused on a modified Langendorff apparatus through the aorta (2 ml/min) with the following solutions: (1) 5 min with Tyrode solution containing (in mM): 130 NaCl; 5.4 KCl; 1 CaCl2; 1 MgCl2; 0.33 Na2HPO4; 10 HEPES, 5.5 glucose (pH adjusted to 7.4 with NaOH); (2) 10 min with Tyrode solution with zero Ca2+; (3) 20 min with Tyrode solution containing 0.03 mM Ca2+, 20 mM taurine, 0.1% bovine serum albumin (BSA; Fraction V, Sigma Chemicals Co., St. Louis, MO, USA) and 73.7 U/ml type II collagenase (Worthington Co. Ltd., Freehold, NJ, USA); and (4) 5.5 min with Kraftbrühe (KB) solution containing (in mM) 100 K+-glutamate; 10 K+-aspartate; 25 KCl; 10 KH2PO4, 2 MgSO4; 20 creatine base; 0.5 EGTA; 5 HEPES; 0.1% BSA, 20 glucose (pH to 7.4 with KOH) (Isenberg and Klockner, 1982). During cell isolation solutions were maintained at 37 ± 1 °C and were equilibrated with 100% O2. At the end of the perfusion the right ventricular free wall was dissected from the heart and placed in “KB” solution. The ventricular tissue was triturated gently with a Pasteur pipette for 10–15 min to free individual ventricular myocytes. Rod-shape single myocytes were then collected and stored in “KB” solution at 4 °C until use[6]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed following topical application. Metabolism / Metabolites 4-Hydroxytamoxifen has known human metabolites that include 3,4-Dihydroxy-Tamoxifen, Tamoxifen 4-O-sulfate, Tamoxifen 4-O-glucuronide, and Endoxifen. 4-Hydroxytamoxifen is a known human metabolite of tamoxifen. |
| 参考文献 |
[1]. en.wikipedia.org/wiki/Afimoxifene
[2]. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008 Mar;7(3):670-8. [3].J Pharmacol Sci. 2015 Jul;128(3):144-9. [4]. Breast Cancer Res Treat. 2007 Dec;106(3):389-97. [5]. Cancer Res. 2013 Jul 15; 73(14): 4395–4405. [6]. Eur J Pharmacol. 2010 Mar 10;629(1-3):96-103. |
| 其他信息 |
Afimoxifene is a tertiary amino compound that is tamoxifen in which the phenyl group which is in a Z- relationship to the ethyl substituent is hydroxylated at the para- position. It is the active metabolite of tamoxifen. It has a role as an antineoplastic agent, an estrogen receptor antagonist and a metabolite. It is a tertiary amino compound and a member of phenols. It is functionally related to a tamoxifen.
Afimoxifene (4-Hydroxytamoxifen, trade name TamoGel) is a new estrogen inhibitor under investigation for a variety of estrogen-dependent conditions, including cyclic breast pain and gynecomastia. TamoGel is formulated using Enhanced Hydroalcoholic Gel (EHG) Technology. This technology enables percutaneous delivery of drugs that cannot be delivered orally. It is being developed by Ascent Therapeutics. 4-Hydroxytamoxifen has been reported in Penicillium aurantiacobrunneum with data available. Afimoxifene is a tamoxifen metabolite with both estrogenic and anti-estrogenic effects. Afimoxifene has a higher affinity for the estrogen receptor than tamoxifen, and functions as an antagonist in breast cancer cells. 4-Hydroxytamoxifen (Afimoxifene) is a metabolite of Tamoxifen. Afimoxifene (4-hydroxytamoxifen) is a selective estrogen receptor modulator which is the active metabolite of tamoxifen. Afimoxifene is a transdermal gel formulation and is being developed by Ascend Therapeutics, Inc. under the trademark TamoGel. (Wikipedia) Drug Indication For the potential treatment of menstrual-cycle related mastalgia, fibrocystic breast disease, breast disease, gynecomastia and Keloid scarring. Mechanism of Action Afimoxifene binds to estrogen receptors (ER), inducing a conformational change in the receptor. This results in a blockage or change in the expression of estrogen dependent genes. |
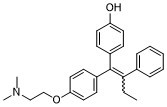
| 分子式 |
C26H29NO2
|
|---|---|
| 分子量 |
387.51
|
| 精确质量 |
387.22
|
| 元素分析 |
C, 80.59; H, 7.54; N, 3.61; O, 8.26
|
| CAS号 |
68392-35-8
|
| 相关CAS号 |
4-Hydroxytamoxifen;68047-06-3;(E)-4-Hydroxytamoxifen;174592-47-3
|
| PubChem CID |
449459
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
| 密度 |
1.092
|
| 熔点 |
135-144°C
|
| LogP |
5.701
|
| tPSA |
32.7
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
493
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(CCOC1C=CC(/C(=C(/C2C=CC=CC=2)\CC)/C2C=CC(O)=CC=2)=CC=1)C
|
| InChi Key |
TXUZVZSFRXZGTL-OCEACIFDSA-N
|
| InChi Code |
InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25+
|
| 化学名 |
4-[1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-phenol
|
| 别名 |
(E/Z)-4-Hydroxytamoxifen; 4 hydroxytamoxifene ; 4Hydroxytamoxifen; paraHydroxytamoxifen; 4Monohydroxytamoxifen; Hydroxytamoxifen; Afimoxifene; Tamogel 4Hydroxytamoxifen; trans4Hydroxytamoxifen; Tamoxifen metabolite B; 4hydroxytamoxifen.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~83.33 mg/mL (~215.04 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.37 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5806 mL | 12.9029 mL | 25.8058 mL | |
| 5 mM | 0.5161 mL | 2.5806 mL | 5.1612 mL | |
| 10 mM | 0.2581 mL | 1.2903 mL | 2.5806 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04009044 | Recruiting | Drug: Afimoxifene Procedure: Core Biopsy |
Cancer Survivor Ductal Breast Carcinoma In Situ |
Northwestern University | February 17, 2020 | Phase 2 |
| NCT03063619 | Completed Has Results | Drug: Afimoxifene Other: Laboratory Biomarker Analysis |
Mammographically Dense Breast | M.D. Anderson Cancer Center | January 30, 2018 | Phase 2 |
| NCT03199963 | Terminated Has Results | Drug: 4-OH tamoxifen Drug: Placebo |
Mammographic Breast Density | BHR Pharma, LLC | August 21, 2017 | Phase 3 |
| NCT02993159 | Recruiting | Drug: Afimoxifene Other: Laboratory Biomarker Analysis |
Ductal Breast Carcinoma In Situ Estrogen Receptor Positive |
Northwestern University | May 31, 2017 | Phase 2 |