| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 25g |
|
||
| Other Sizes |
|

| 靶点 |
ENaC; uTPA; polycystin-2 (TRPP2)
Epithelial Sodium Channels (ENaCs) [1] - Urokinase-type Plasminogen Activator Receptor (uTPA)[2] |
|---|---|
| 体外研究 (In Vitro) |
阿米洛利还诱导 P13K(磷脂酰肌醇 3 激酶)和 PDK-1(磷酸肌醇依赖性激酶 1)激酶以及 PTEN(10 号染色体上删除的磷酸酶和张力蛋白同源物)和 PP1 α 磷酸酶的去磷酸化。阿米洛利通过与 ATP 竞争来抑制激酶和磷酸酶的磷酸化。阿米洛利本身很少或没有细胞毒性,可增强 TRAIL 诱导的细胞凋亡。阿米洛利阻止碱化并同时抑制细胞增殖。阿米洛利直接抑制 EGF 受体的自身磷酸化。阿米洛利显着地将力、+dF/dt 和 -dF/dt 的恢复分别提高到最大 39%、88% 和 78%。阿米洛利是一种常用的 Na+/H+ 交换抑制剂,可快速抑制体内佛波酯刺激的蛋白质磷酸化和体外蛋白激酶 C 介导的磷酸化,两者的效力与阿米洛利抑制 Na+/H+ 交换的效力相似。阿米洛利可阻断佛波酯诱导的 HL-60 细胞粘附(粘附是分化状态的一种特性),但二甲基阿米洛利(以及乙基异丙基阿米洛利,另一种非常有效的阿米洛利类似物)则不会。在不同浓度的细胞外钠存在下,阿米洛利可抑制兔完整近端小管悬浮液的哇巴因敏感耗氧率 (QO2)。细胞测定:阿米洛利阻断 δβγ 通道,IC50 为 2.6 μM (58, 71, 75, 134, 148)。阿米洛利对 δβγ ENaC 的 Ki 是 αβγ 通道的 26 倍(αβγ ENaC 为 0.1 μM)。与 αβγ 通道相比,阿米洛利对 δβγ ENaC 的阻断更加依赖于电压。阿米洛利对 δαβγ 通道的 Ki 在 -120 和 +80 mV 时分别为 920 和 13.7 μM,与 αβγ 和 δβγ 通道的 Ki 显着不同。阿米洛利是一种相对选择性的上皮钠通道 (ENaC) 抑制剂,IC50(离子通道抑制达到 50% 所需的浓度)在 0.1 至 0.5 μM 的浓度范围内。阿米洛利是一种相对较差的 Na+/H+ 交换器 (NHE) 抑制剂,在低外部 [Na+] 存在下,IC50 低至 3 μM,但在高 [Na+] 存在下,IC50 高达 1 mM。阿米洛利是一种更弱的 Na+/Ca2+ 交换剂 (NCX) 抑制剂,IC50 为 1 mM。阿米洛利 (1 μM) 和亚微摩尔剂量的苯扎米尔 (30 nM) 已知可抑制 ENaC,通过阻断 ENaC 蛋白的活性来抑制对灌注压增加的肌源性血管收缩反应。阿米洛利以已知对血管平滑肌细胞 (VSMC) 中 ENaC (1.5 μM) 相对特异的剂量完全抑制 Na+ 内流。
对培养的足细胞进行不同浓度的二水合盐酸阿米洛利(MK 870)处理,该药物可显著抑制足细胞中的uTPA活性,表现为纤溶酶原向纤溶酶的裂解减少。此外,二水合盐酸阿米洛利(MK 870)可在mRNA和蛋白水平上上调足细胞特异性蛋白(podocin和nephrin)的表达,这些蛋白对维持肾小球滤过屏障的完整性至关重要。该药物还可降低脂多糖(LPS)刺激下足细胞中促炎细胞因子(TNF-α、IL-6)的分泌[2] |
| 体内研究 (In Vivo) |
上皮钠通道 (ENaCs) 的强抑制剂是阿米洛利盐酸盐二水合物。注射后十五至三十分钟内,阿米洛利在血浆中显着浓缩。与基线读数 (n = 7) 相比,2 mg/kg 剂量的盐酸阿米洛利二水合物对血压、心率、肠系膜血管阻力或后躯血管阻力没有影响。与基线值相比,盐酸阿米洛利二水合物在 2 小时内对心率 (-10±6 bpm/min) 和动脉压 (-1±1 mmHg) 造成的变化非常小。根据结果,后区 (AP) 中的 c-Fos 激活表现出剂量相关的反应模式。盐酸阿米洛利二水合物,即使在最低剂量 0.1 mg/kg 下,c-Fos 标记的神经元数量也与对照大鼠存在统计差异,p<0.01 水平[1]。
对成年雄性Sprague-Dawley大鼠进行腹腔注射二水合盐酸阿米洛利(MK 870),剂量为10 mg/kg。脑组织免疫组织化学染色显示,给药2小时后,最后区(AP)的c-Fos阳性细胞显著增加。定量分析表明,AP区c-Fos阳性神经元数量约为对照组的3倍。这种激活具有AP区特异性,在邻近脑区(如孤束核)未观察到c-Fos表达的显著变化[1] |
| 酶活实验 |
uTPA活性检测:用二水合盐酸阿米洛利(MK 870)处理足细胞后裂解细胞,将细胞裂解液与纤溶酶原底物和显色剂共同孵育,在不同时间点测定405 nm处的吸光度,计算uTPA活性。该实验表明,二水合盐酸阿米洛利(MK 870)以浓度依赖的方式抑制uTPA活性[2]
|
| 细胞实验 |
本研究探讨了尿激酶型纤溶酶原激活物受体抑制剂阿米洛利在降低蛋白尿中的作用机制。对足细胞进行复苏以促进其增殖,并观察其形态变化。在体外实验中,建立了对照组、脂多糖组和脂多糖+阿米洛利组。用流式细胞仪检测足突细胞中尿激酶型纤溶酶原激活物受体(uPAR)的表达,用transwell迁移试验检测细胞运动[2]。
足细胞培养与处理:将足细胞接种到6孔板中培养至汇合,随后用浓度为1 μM、10 μM和100 μM的二水合盐酸阿米洛利(MK 870)处理24小时,在处理结束前6小时向培养基中加入1 μg/mL的LPS以诱导炎症反应[2] - 蛋白表达检测:提取处理后足细胞的总蛋白,经SDS-PAGE分离后转移至硝酸纤维素膜,用抗podocin、nephrin和β-肌动蛋白(内参)的一抗孵育,再加入二抗,通过化学发光法显影蛋白条带,并对条带密度进行定量分析[2] - mRNA表达检测:提取足细胞总RNA,逆转录为cDNA,使用podocin、nephrin和GAPDH(内参)的特异性引物进行实时荧光定量PCR(qPCR),采用2^(-ΔΔCt)法计算相对mRNA表达水平[2] - 细胞因子检测:按照标准流程,采用酶联免疫吸附试验(ELISA)检测细胞培养上清液中TNF-α和IL-6的浓度[2] |
| 动物实验 |
1 mg/kg/day; subcutaneous
Rats Epithelial sodium channels (ENaCs) are strongly expressed in the circumventricular organs (CVOs), and these structures may play an important role in sensing plasma sodium levels. Here, the potent ENaC blocker amiloride was injected intraperitoneally in rats and 2h later, the c-Fos activation pattern in the CVOs was studied. Amiloride elicited dose-related activation in the area postrema (AP) but only ~10% of the rats showed c-Fos activity in the organum vasculosum of the lamina terminalis (OVLT) and subfornical organ (SFO). Tyrosine hydroxylase-immunoreactive (catecholamine) AP neurons were activated, but tryptophan hydroxylase-immunoreactive (serotonin) neurons were unaffected. The AP projects to FoxP2-expressing neurons in the dorsolateral pons which include the pre-locus coeruleus nucleus and external lateral part of the parabrachial nucleus; both cell groups were c-Fos activated following systemic injections of amiloride. In contrast, another AP projection target--the aldosterone-sensitive neurons of the nucleus tractus solitarius which express the enzyme 11-β-hydroxysteriod dehydrogenase type 2 (HSD2) were not activated. As shown here, plasma concentrations of amiloride used in these experiments were near or below the IC50 level for ENaCs. Amiloride did not induce changes in blood pressure, heart rate, or regional vascular resistance, so sensory feedback from the cardiovascular system was probably not a causal factor for the c-Fos activity seen in the CVOs. In summary, amiloride may have a dual effect on sodium homeostasis causing a loss of sodium via the kidney and inhibiting sodium appetite by activating the central satiety pathway arising from the AP.[1] In the in vivo test, the urine protein volume of the model was detected at 24 h using Coomassie brilliant blue staining and the morphological changes of the podocytes were detected with immunofluorescence. The protein expression rate of uPAR in the lipopolysaccharide group was significantly higher than those in the control and lipopolysaccharide + amiloride groups (P < 0.05). The viability of cells in the lipopolysaccharide group was significantly higher than those in the control and lipopolysaccharide + amiloride groups (P < 0.05). Compared with the urine protein level in the control group at 24 h, the level in the lipopolysaccharide group increased significantly (P < 0.05), whereas compared with the urine protein level in the lipopolysaccharide group, the level in the lipopolysaccharide + amiloride group decreased (P < 0.05). uPAR expression was significantly downregulated, and the fusion of the podocyte-specific skelemin synaptopodin on the glomerulus podocytes was significantly decreased in the lipopolysaccharide + amiloride group. These results suggest that amiloride is able to reduce cell motility and thus lower proteinuria by inhibiting the expression of uPAR in podocytes.[2] Adult male Sprague-Dawley rats (250-300 g) were housed under standard laboratory conditions with free access to food and water. After acclimatization for 1 week, rats were randomly divided into control group and Amiloride HCl dihydrate (MK 870) treatment group. The treatment group received a single intraperitoneal injection of the drug at a dose of 10 mg/kg, while the control group received an equal volume of normal saline[1] - Two hours after administration, rats were anesthetized with pentobarbital sodium and perfused transcardially with 4% paraformaldehyde. The brain was removed and post-fixed in 4% paraformaldehyde for 24 hours, then dehydrated and embedded in paraffin. Serial coronal sections (5 μm) containing the area postrema were cut and subjected to immunohistochemical staining for c-Fos[1] |
| 参考文献 |
|
| 其他信息 |
Amiloride hydrochloride dihydrate is a hydrate that is the dihydrate of amiloride hydrochloride. It has a role as a diuretic and a sodium channel blocker. It contains an amiloride hydrochloride.
Amiloride Hydrochloride is the hydrochloride salt of amiloride, a synthetic pyrazine derivative with antikaliuretic and diuretic properties. Amiloride inhibits sodium channels located in the distal tubules and collecting ducts of the kidney, thereby preventing the absorption of sodium and increasing its excretion along with water, to produce naturesis. In response to the hypernatremic conditions in the kidney, the plasma membrane becomes hyperpolarized and electrochemical forces are reduced, which then prevents the excretion of potassium and hydrogen into the lumen. A pyrazine compound inhibiting SODIUM reabsorption through SODIUM CHANNELS in renal EPITHELIAL CELLS. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with DIURETICS to spare POTASSIUM loss. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th ed, p705) Amiloride HCl dihydrate (MK 870) is a well-known ENaC blocker. This study reveals that its blockade of ENaCs in the area postrema can induce c-Fos activation, suggesting a potential role in regulating osmotic balance and related physiological processes[1] - This study is the first to identify Amiloride HCl dihydrate (MK 870) as a uTPA inhibitor. Its ability to protect podocyte function and reduce inflammatory responses provides a potential therapeutic strategy for kidney diseases associated with proteinuria[2] |
| 分子式 |
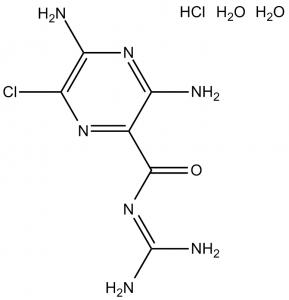
C6H8CLN7O.HCL.2H2O
|
|
|---|---|---|
| 分子量 |
302.12
|
|
| 精确质量 |
301.045
|
|
| 元素分析 |
C, 23.85; H, 4.34; Cl, 23.47; N, 32.45; O, 15.89
|
|
| CAS号 |
17440-83-4
|
|
| 相关CAS号 |
Amiloride hydrochloride;2016-88-8;Amiloride;2609-46-3
|
|
| PubChem CID |
68540
|
|
| 外观&性状 |
Typically exists as Off-white to yellow solids at room temperature
|
|
| 沸点 |
628.1ºC at 760 mmHg
|
|
| 熔点 |
>240℃
|
|
| 闪点 |
333.7ºC
|
|
| 蒸汽压 |
1.08E-15mmHg at 25°C
|
|
| LogP |
1.944
|
|
| tPSA |
175.25
|
|
| 氢键供体(HBD)数目 |
7
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
279
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C(N([H])[H])=NC(=C(C(/N=C(\N([H])[H])/N([H])[H])=O)N=1)N([H])[H].Cl[H].O([H])[H].O([H])[H]
|
|
| InChi Key |
LTKVFMLMEYCWMK-UHFFFAOYSA-N
|
|
| InChi Code |
1S/C6H8ClN7O.ClH.2H2O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11;;;/h(H4,8,9,13)(H4,10,11,14,15);1H;2*1H2
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.27 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3099 mL | 16.5497 mL | 33.0994 mL | |
| 5 mM | 0.6620 mL | 3.3099 mL | 6.6199 mL | |
| 10 mM | 0.3310 mL | 1.6550 mL | 3.3099 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。