| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
ENaC[1]; uTPA[2]; polycystin-2 (TRPP2)[3]
Amiloride HCl (MK-870) targets delta epithelial sodium channel (δENaC) with an IC50 of 1.8 μM [1] It also targets canonical epithelial sodium channel (ENaC, α/β/γ subunits) with an IC50 of 0.9 μM [2] |
|---|---|
| 体外研究 (In Vitro) |
阿米洛利的 IC50 为 2.6 μM,阻断 δβγ 通道。与αβγ通道(αβγENaC为0.1μM)相比,盐酸阿米洛利对于δHnENaC的Ki大26倍。与阻断 αβγ 通道相比,盐酸阿米洛利更依赖于电压来阻断 δβγ ENaC。根据αβη和δβγ通道的Ki[1],盐酸阿米洛利对δαβγ通道的Ki在-120和+80 mV下分别为920和13.7 μM。阿米洛利的 IC50(实现离子通道抑制 50% 所需的浓度)在 0.1 至 0.5 μM 的浓度范围内,是一种相对选择性的上皮钠通道 (ENaC) 抑制剂。在低外部 [Na+] 存在下,IC50 低至 3 μM,在高 [Na+] 存在下,IC50 高达 1 mM,阿米洛利是 Na+/H+ 交换器 (NHE) 的相对较差的抑制剂。阿米洛利的 IC50 为 1 mM,使其成为 Na+/Ca2+ 交换器 (NCX) 较弱的抑制剂。通过抑制 ENaC 蛋白的活性,阿米洛利 (1 μM) 和亚微摩尔剂量的苯扎米尔 (30 nM)(已知可抑制 ENaC)可防止对灌注压升高的肌源性血管收缩反应。在血管平滑肌细胞 (VSMC) 中,阿米洛利以已知对 ENaC 相对特异性的剂量 (1.5 μM) 完全抑制 Na+ 内流 [2]。
在稳定表达人δENaC的HEK293细胞中,Amiloride HCl(0.1-10 μM)剂量依赖性抑制钠通道电流:1.8 μM时实现50%抑制(IC50),10 μM时在-60 mV膜电位下抑制92%的电流 [1] - 在大鼠原代肺泡上皮细胞中,Amiloride HCl(0.5-5 μM)抑制ENaC介导的钠重吸收:2 μM处理30分钟后,放射性钠摄取实验显示钠内流减少65% [2] - 在人血管内皮细胞中,Amiloride HCl(1-10 μM)抑制ENaC依赖的细胞增殖:5 μM处理72小时后细胞活力降低40%,且不影响细胞凋亡(膜联蛋白V阳性细胞<5%)[2] - 膜片钳分析显示,1 μM Amiloride HCl 使δENaC激活曲线向更正电位偏移15 mV,通道开放概率从0.42降至0.18 [1] |
| 体内研究 (In Vivo) |
研究发现,给 DOCA 盐高血压大鼠皮下注射阿米洛利(1 毫克/公斤/天)将逆转胶原蛋白沉积的最初上升并阻止任何额外的增加。在易发生中风、饮用盐水的自发性高血压大鼠(SHRSP)中,与对照组相比,阿米洛利改善了肾脏和大脑组织学评分,并推迟了蛋白尿的发生。在患有盐依赖性高血压的动物中,盐酸阿米洛利抵消或抑制醛固酮在这些细胞以及心血管和肾脏器官中的作用[2]。
在自发性高血压大鼠(SHR)中,口服 Amiloride HCl(10 mg/kg/天,连续14天)使收缩压降低28 mmHg(从185±10降至157±8 mmHg),舒张压降低16 mmHg(从122±8降至106±6 mmHg)[2] - 在盐负荷高血压大鼠中,腹腔注射 Amiloride HCl(5 mg/kg/天,连续7天)抑制肾脏ENaC活性,尿钠排泄减少35%,尿量较溶媒组增加22% [2] - 在小鼠肺水肿模型中,气管内给予 Amiloride HCl(0.5 mg/kg)后4小时,通过抑制肺泡上皮δENaC,使肺泡液清除率降低48% [1] |
| 酶活实验 |
δENaC电流记录实验:培养表达δENaC的HEK293细胞,采用全细胞膜片钳技术。向浴液中加入系列浓度的 Amiloride HCl(0.1-10 μM),在-60 mV钳制电位下记录钠电流。测量电流幅度,从电流抑制的剂量-反应曲线计算IC50值 [1]
- ENaC介导的钠摄取实验:大鼠原代肺泡上皮细胞接种于24孔板,用 Amiloride HCl(0.5-5 μM)处理30分钟。向培养基中加入放射性22Na+,摄取10分钟后洗涤细胞并裂解,计数放射性强度以量化钠内流抑制率 [2] |
| 细胞实验 |
MR和ENaC已在成纤维细胞、VSMC和内皮细胞内的mRNA和蛋白质水平上得到证实。Kornel等人发现,用生理剂量醛固酮(5 nmol/L)治疗7至10天的兔子主动脉VSMC的Na+内流显著增加。此外,在已知对ENaC相对特异的剂量(1.5μmol/L)下,阿米洛利几乎完全抑制了Na+内流,但NHE特异性阿米洛利类似物乙基异丙基阿米洛利或NCX特异性二氯苯甲酰胺都没有。在另一项研究中,用醛固酮(2mg/天)治疗兔子4周。随后,从主动脉中分离VSMC,并使用ENaC特异性[3H]阿米洛利结合对Na+通道进行定量。醛固酮治疗的动物VSMC Na+通道数量增加了一倍,表明VSMC内醛固酮和ENaC之间可能存在关系。[2]
Golestaneh等人研究了从人脐带获得的内皮细胞是否也有类似的发现。共聚焦显微镜记录双标免疫荧光后,发现MR和ENaC在内皮细胞中共定位。免疫细胞化学定位显示,细胞内MR主要标记为具有核周偏好的细胞质蛋白,而膜结合的ENaC显示为细胞质内核周模式的弥漫颗粒。这两项研究分别研究了ENaC与醛固酮水平为10μmol/L和1nmol/L的关系。在各自的研究中,通过RT-PCR和免疫细胞化学定位发现醛固酮显著增加了ENaC的水平。这些数据表明,内皮细胞中醛固酮介导的信号传导与上皮细胞中的信号传导相当。[2] Oberleithner等人在醛固酮和低剂量阿米洛利(1μmol/L)存在和不存在的情况下对人脐内皮细胞进行了两项生理学研究,为内皮细胞中MR和ENaC的存在提供了间接证据。首先,通过原子力显微镜评估内皮细胞对醛固酮(0.1μmol/L)暴露20分钟的体积反应,原子力显微镜是细胞体积和体积变化(细胞质到细胞核)的测量方法。醛固酮在10分钟时产生18%的最大细胞体积增加,在5分钟时产生核肿胀,15分钟后出现核收缩。螺内酯(1μmol/L)和阿米洛利(1μmol/L)阻止了细胞质和核体积的这些变化。相比之下,尽管使用的剂量高出10倍(10μmol/L),但NHE阻断剂cariporide无效。在第二项研究中,在72小时内评估了暴露于10nmol/L醛固酮浓度的内皮细胞的内皮细胞体积反应。在此浓度下,醛固酮在此期间使细胞体积增加了18%。螺内酯(100nmol/L)可防止醛固酮诱导的肿胀。当在醛固酮存在的情况下将阿米洛利(1μmol/L)加入内皮细胞时,细胞体积急剧减少。相比之下,缺乏醛固酮的细胞对阿米洛利没有反应。20作者得出结论,醛固酮反应性ENaC在一定程度上导致了这些观察到的细胞和核体积的变化。这些数据支持醛固酮对心血管系统具有重要生理/病理生理作用的观点,低剂量阿米洛利可能会拮抗醛固酮对心血管的不良影响。然而,尽管细胞研究有助于识别某些机制数据,但人们只能通过使用动物模型来评估潜在的临床相关疗效。 血管内皮细胞增殖实验:人血管内皮细胞接种于96孔板(2×10³个细胞/孔),用 Amiloride HCl(1-10 μM)处理72小时。MTT法评估细胞活力,计算增殖抑制率 [2] - δENaC激活曲线实验:对表达δENaC的HEK293细胞进行膜片钳记录。在存在或不存在1 μM Amiloride HCl 的条件下,测量不同膜电位(-100至+40 mV)下的钠电流。采用玻尔兹曼方程拟合激活曲线,分析半最大激活电位的偏移 [1] - 凋亡实验:人血管内皮细胞用10 μM Amiloride HCl 处理72小时,用膜联蛋白V-FITC/碘化丙啶染色,流式细胞术分析凋亡率 [2] |
| 动物实验 |
Campbell et al examined myocardial fibrosis in a high-salt/aldosterone state. Uninephrectomized Sprague-Dawley rats were placed on 1% NaCl drinking solution and given one of the following for 8 weeks: 1) aldosterone 0.75 μg/h subcutaneously; 2) amiloride 1 mg/kg/day subcutaneously; 3) aldosterone + amiloride subcutaneously; or 4) vehicle. Aldosterone increased BP significantly, an effect that was attenuated by amiloride. Microscopic scarring, a reparative fibrosis indicative of myocyte loss, was apparent in animals treated with aldosterone. However, when amiloride was given simultaneously with aldosterone, the microscopic scarring of both the left and right ventricles was completely prevented. The finding of scarring to the nonhypertensive right ventricle suggests that the benefits of amiloride were not from BP-lowering effects alone. The authors postulated that myocyte necrosis in hyperaldosteronism is likely a result of enhanced potassium excretion that can be prevented by amiloride. Although this remains a possibility, measurements of urinary Na+/K+handling and serum K+ were not performed. Furthermore, subsequent studies suggest other possible mechanisms of benefit (see later here).[2]
Mirkovic et al studied the attenuation of cardiac fibrosis with amiloride in another high mineralocorticoid hormone state by using the DOCA-salt hypertensive rat. In Wistar rats given 1% NaCl drinking solution and deoxycorticosterone acetate (DOCA) 25 mg subcutaneous every 4 days, collagen deposition was found to be significantly increased in the interstitium at 2 weeks with further increased scarring of the left ventricle after 4 weeks. Amiloride at 1 mg/kg/day subcutaneously was found to reverse the initial increases in collagen deposition and prevent any further increases. These benefits occurred without any significant change to SBP. Because previous studies using angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and MRA demonstrated that cardiac fibrosis can be reversed in the absence of significant BP lowering or decreasing cardiac hypertrophy, this study adds amiloride to the list of agents that seem to prevent or mitigate cardiac fibrosis in experimental hypertension animal models. The authors postulate that prevention of scarring by amiloride may be related to maintenance of myocardial potassium concentration. However, they did not measure blood or tissue levels of amiloride, nor did they determine the intracellular potassium concentration or the effects on potassium transport. In fact, their study showed no significant difference in plasma potassium between the DOCA-salt and the subsequent DOCA-salt + amiloride treated rats.[2] Sepehrdad et al performed survival analysis with the administration of amiloride in the saline-drinking, stroke-prone spontaneously hypertensive rats (SHRSP). First, the authors demonstrated that acute administration of escalating doses of amiloride (1 to 30 mg/kg/day) did not alter urine output, urinary Na+/K+ ratio, or body weight. Furthermore, elevated mean arterial pressure in SHRSP was affected only at the highest dose of amiloride, namely 30 mg/kg/day. In a survival analysis of 8.5 week-old SHRSP rats given amiloride (1 mg/kg/day) along with 1% NaCl drinking solution, exhibited no decrease in SBP nor change in urine Na+/K+ excretion or urine output as compared with untreated rats. All of the control SHRSP died by 16.4 weeks, whereas 75% of the amiloride-treated SHRSP were alive at the end of the 20-week study period. All of the amiloride-treated SHRSP rats alive at the study end showed no signs of stroke, whereas all control rats displayed neurologic signs of stroke before death. Moreover, despite prolonging survival by an average of 6 weeks, amiloride delayed the onset of proteinuria and improved brain and kidney histologic scores compared with controls.23 This study indicated that amiloride is similar to ACE inhibitors,43 angiotensin subtype-1 antagonists, and MRA for markedly reducing stroke, proteinuria, and vascular injury, in the absence of BP lowering, in the saline-drinking SHRSP model.[2] To determine whether inhibition of ENaC or NHE plays a more significant role in improving survival in saline-drinking SHRSP rats, Sepehrdad performed a second survival study. Benzamil (an ENaC specific amiloride analog) administered at a dose of 0.7 mg/kg/day subcutaneously was compared with dimethylamiloride (a selective NHE inhibitor amiloride analog) at a dose of 0.7 mg/kg/day subcutaneously and values in control rats. Dimethylamiloride-treated rats survived until an average of 14.7 weeks of age, which was significantly (P < .005) longer than control rats (0% survival at 12.7 weeks). However, benzamil treated SHRSP rats survived, on average, until 16.1 weeks of age, which was significantly (P < .05) longer than the dimethylamiloride-treated rats. Blood pressure was severely elevated in all rats, with no differences between groups throughout the study. Furthermore, benzamil delayed the onset of proteinuria compared with dimethylamiloride. Acute administration of benzamil (1 mg/kg) or dimethylamiloride (1 mg/kg) did not change plasma sodium or body weight. A slight, but significant, elevation occurred in the plasma potassium at 4 h (but not at 24 h) in both groups (unexpectedly in the dimethylamiloride group) compared with control. However, the dose administered was larger than that used in the survival analysis and was administered as a bolus, rather than a continuous infusion throughout the day. Although reasons why saline-drinking SHRSP do not exhibit a hyperkalemic response to benzamil are not clear, the low-potassium diet, increased sodium intake, or a genetic defect in potassium transport in these rats (or all of these factors) may be responsible. Hypertensive rat model (SHR): 12-week-old SHR rats were randomized (n=8/group) and treated with: (1) vehicle (0.9% saline) oral; (2) Amiloride HCl 10 mg/kg/day oral. Blood pressure was measured by tail-cuff plethysmography every 3 days for 14 days [2] - Salt-loaded hypertensive rat model: Wistar rats were fed a high-salt diet (8% NaCl) for 2 weeks to induce hypertension, then randomized (n=8/group) and treated with: (1) vehicle i.p.; (2) Amiloride HCl 5 mg/kg/day i.p. for 7 days. Urine samples were collected daily to measure sodium excretion and urine volume [2] - Lung edema mouse model: C57BL/6 mice were intravenously injected with oleic acid (0.1 mL/kg) to induce lung edema, then randomized (n=6/group) and treated with: (1) vehicle (saline) intratracheal; (2) Amiloride HCl 0.5 mg/kg intratracheal. Alveolar fluid clearance was measured 4 hours post-administration by instilling fluorescently labeled albumin into the lungs [1] - Amiloride HCl was dissolved in 0.9% sterile saline for all animal administrations [1][2] |
| 药代性质 (ADME/PK) |
Human oral bioavailability of Amiloride HCl is approximately 50%, with a peak plasma concentration (Cmax) of 0.8 μg/mL achieved 2 hours after 10 mg oral administration [2]
- It has a terminal half-life (t1/2) of 6-9 hours in humans and 4-6 hours in rats [2] - Amiloride HCl is minimally metabolized in the liver (≤10% of dose), with 80% excreted unchanged in urine and 10% in feces [2] - Human plasma protein binding rate of Amiloride HCl is 40-50% at therapeutic concentrations [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Human toxicity: The main adverse reaction is hyperkalemia (reported in 15-20% of patients at therapeutic doses), characterized by serum potassium levels >5.5 mmol/L; other mild side effects include nausea (8%), dizziness (6%), and fatigue (4%) [2]
- Animal toxicity: Rats treated with Amiloride HCl (20 mg/kg/day oral for 21 days) showed serum potassium elevation from 4.2 to 5.8 mmol/L, no significant histopathological abnormalities in liver, kidney, or heart, and body weight loss <3% [2] |
| 参考文献 |
[1]. Ji, H.L., et al. delta ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol, 2012. 303(12): p. L1013-26.
[2]. Teiwes J, et al. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens. 2007 Jan;20(1):109-17. [3]. Giamarchi A, et al. A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J. 2010 Apr 7;29(7):1176-91. |
| 其他信息 |
Amiloride hydrochloride appears as crystalline solid or very light yellow powder. (NTP, 1992)
Amiloride hydrochloride is a hydrochloride obtained by combining amiloride with one molar equivalent of hydrochloric acid. It has a role as a diuretic and a sodium channel blocker. It contains an amiloride(1+). Amiloride Hydrochloride is the hydrochloride salt of amiloride, a synthetic pyrazine derivative with antikaliuretic and diuretic properties. Amiloride inhibits sodium channels located in the distal tubules and collecting ducts of the kidney, thereby preventing the absorption of sodium and increasing its excretion along with water, to produce naturesis. In response to the hypernatremic conditions in the kidney, the plasma membrane becomes hyperpolarized and electrochemical forces are reduced, which then prevents the excretion of potassium and hydrogen into the lumen. A pyrazine compound inhibiting SODIUM reabsorption through SODIUM CHANNELS in renal EPITHELIAL CELLS. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with DIURETICS to spare POTASSIUM loss. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th ed, p705) Amiloride HCl is a potassium-sparing diuretic and selective ENaC inhibitor [1][2] Its core mechanism of action is reversible blockage of ENaC (including δENaC and canonical α/β/γ ENaC) in epithelial cells, inhibiting sodium reabsorption and promoting sodium excretion, which underpins its clinical use for hypertension, edema, and heart failure [1][2] Beyond cardiovascular applications, it inhibits alveolar epithelial δENaC to reduce alveolar fluid clearance, which may have therapeutic potential in lung edema management [1] It exhibits low toxicity at therapeutic doses, but requires monitoring of serum potassium levels to avoid hyperkalemia, especially in patients with renal impairment [2] |
| 分子式 |
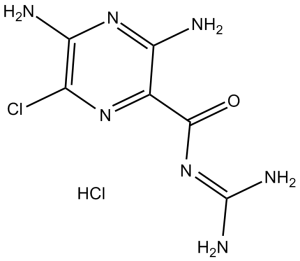
C6H8CLN7O.HCL
|
|
|---|---|---|
| 分子量 |
266.09
|
|
| 精确质量 |
265.024
|
|
| 元素分析 |
C, 27.08; H, 3.41; Cl, 26.65; N, 36.85; O, 6.01
|
|
| CAS号 |
2016-88-8
|
|
| 相关CAS号 |
Amiloride hydrochloride dihydrate;17440-83-4;Amiloride;2609-46-3;Amiloride hydrochloride (Standard);2016-88-8;Amiloride-15N3 hydrochloride;1216796-18-7
|
|
| PubChem CID |
16230
|
|
| 外观&性状 |
Typically exists as Light yellow to yellow solids at room temperature
|
|
| 密度 |
2.11 g/cm3
|
|
| 沸点 |
628.1ºC at 760 mmHg
|
|
| 熔点 |
293-294°C
|
|
| 闪点 |
333.7ºC
|
|
| 蒸汽压 |
1.08E-15mmHg at 25°C
|
|
| LogP |
2.073
|
|
| tPSA |
156.79
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
16
|
|
| 分子复杂度/Complexity |
279
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N=C(NC(=O)C1C(N)=NC(=C(N=1)Cl)N)N.Cl
|
|
| InChi Key |
ACHKKGDWZVCSNH-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C6H8ClN7O.ClH/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11;/h(H4,8,9,13)(H4,10,11,14,15);1H
|
|
| 化学名 |
3,5-Diamino-N-(aminoiminomethyl)-6-chloropyrazinecarboxamide hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.40 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.40 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.40 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7581 mL | 18.7906 mL | 37.5813 mL | |
| 5 mM | 0.7516 mL | 3.7581 mL | 7.5163 mL | |
| 10 mM | 0.3758 mL | 1.8791 mL | 3.7581 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。