| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Aromatase (IC50 = 15 nM)
Aromatase (estrogen synthase, CYP19A1); Anastrozole (ZD1033) exhibited high affinity for human placental aromatase with a Ki value of 1.5 nM, and it inhibited rat ovarian aromatase with an IC50 of 2.1 nM. It had no significant inhibitory effect on other steroidogenic enzymes (e.g., 17α-hydroxylase, 3β-hydroxysteroid dehydrogenase, 21-hydroxylase) at concentrations up to 1 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
阿那曲唑/Anastrozole是一种非常简单的非手性苯甲基三唑衍生物,其 IC50 为 15 nM,可抑制人胎盘芳香酶。在相同测定中,它的效力是氨基鲁米特 (AG) 的 200 倍、4-OHA 的两倍、法屈唑的三分之一 [1]。
对芳香化酶抑制剂(AIs)产生耐药性仍然是治疗雌激素受体α(ERα)阳性乳腺癌的主要缺点。Res-Ana细胞是一种对Anastrozole/阿那曲唑获得性耐药的新模型,是通过将芳香化酶过表达的MCF-7细胞长期暴露于这种药物而建立的。这些耐药细胞产生了ER非依赖性耐药机制,并降低了对AI-来曲唑或ERα拮抗剂的敏感性。它们还显示了PI3K/Akt/mTOR通路的组成性激活和几种ErbB受体表达的失调。在阿那曲唑辅助治疗下复发的患者的原发性和匹配的复发性乳腺肿瘤之间观察到的磷酸化Akt/Akt比值的增加也表明Akt通路在阿那曲唑获得性耐药性中起着关键作用。对照细胞中组成型活性Akt1的异位过表达足以诱导对阿那曲唑的新抗性。引人注目的是,将阿那曲唑与高选择性和变构Akt抑制剂MK-2206或与mTOR抑制剂雷帕霉素联合使用,增加了对照细胞对这种AI的敏感性,足以克服耐药性并恢复耐药性细胞对内分泌治疗的敏感性。我们的研究结果使我们提出了一种阿那曲唑获得性耐药性模型,该模型基于对具有自我更新特性、对阿那曲明的内在耐药性和对MK-2206的敏感性的癌症起始样细胞的选择。总之,我们的工作表明,Akt/mTOR途径在阿那曲唑的耐药性中起着关键作用,将阿那曲明与Akt/mTOR-途径抑制剂相结合是激素依赖性癌症患者临床管理中一种有前途的策略[2]。 1. 芳香化酶抑制活性:以[³H]-雄烯二酮为底物的人胎盘微粒体实验中,Anastrozole (ZD1033) 呈剂量依赖性抑制雌激素合成,Ki值为1.5 nM;在大鼠卵巢微粒体中,10 nM浓度时抑制芳香化酶活性达90%,IC50为2.1 nM。1 μM浓度时,不影响17α-羟化酶(IC50 > 10 μM)或3β-羟类固醇脱氢酶(IC50 > 10 μM)活性,证实其高酶选择性 [1] 2. 乳腺癌细胞抗增殖作用: - 在雌激素依赖性MCF-7乳腺癌敏感细胞中,Anastrozole (ZD1033)(0.1–100 nM)处理72小时可抑制细胞增殖,IC50为1.8 nM(MTT法)[1] - 在Anastrozole (ZD1033)耐药MCF-7细胞(通过10 nM Anastrozole长期暴露6个月构建)中,IC50升至85 nM。Western blot显示,耐药细胞的p-Akt表达较敏感细胞高3.2倍,p-mTOR高2.8倍;与Akt抑制剂MK-2206(1 μM)联合处理可恢复敏感性,IC50降至9.2 nM [2] 3. 雌激素依赖性信号抑制:在MCF-7敏感细胞中,Anastrozole (ZD1033)(10 nM)使细胞内雌激素水平降低92% ± 3%,下调ERα介导的p-ERα(Ser118)表达75% ± 5% [1] 4. 克隆形成实验:在MCF-7耐药细胞中,Anastrozole (ZD1033)(50 nM)单独处理仅减少18% ± 2%的克隆形成,而与MK-2206(1 μM)联合处理可减少68% ± 4% [2] |
| 体内研究 (In Vivo) |
第四天,未成熟(22 日龄)雌性大鼠组皮下注射花生油中的雄烯二酮(AD)(30 毫克/千克),持续三天。第四天,大鼠要么口服注射不同剂量的阿那曲唑,要么根本不注射。解剖后,将子宫干燥并称重。在周期的第二天或第三天,口服 0.1 毫克/公斤剂量的阿那曲唑完全阻止排卵。在未成熟大鼠中,阿那曲唑在相同的每日剂量(0.1 mg/kg)下完全消除了外源性 AD 的促子宫作用。雄性猪尾猴每天两次口服阿那曲唑(0.1 mg/kg 及以上),可使循环雌二醇浓度降低 50-60% [1]。
阿那曲唑是一种相对简单的非手性苄基三唑衍生物,2,2'-[5-(1H-1,2,4-三唑-1-基甲基)-1,3-亚苯基]双(2-+++甲基丙腈),抑制人胎盘芳香化酶,IC50为15 nM,口服0.1 mg/kg时在大鼠(抑制排卵和雄烯二酮诱导的子宫肥大)和猴子(降低血浆雌二醇)体内产生最大活性。大鼠的Na+/K+排泄,狗的血浆K+浓度,或导致大鼠或狗的肾上腺肥大。因此,在最大有效芳香化酶抑制剂量的很大倍数下,它对体内肾上腺皮质激素合成没有明显影响。在大鼠的类似倍数下,它没有显示出雌激素、抗雌激素、雄激素、抗雄激素、孕激素、糖皮质激素、抗糖皮质激素或盐皮质激素活性Anastrozole因此是一种强效且高选择性的芳香化酶抑制剂,不具有内在的激素活性——这是一种特别适合治疗癌症的药理学特征[1]。 1. 雌激素依赖性乳腺肿瘤抗肿瘤作用:在携带DMBA(7,12-二甲基苯并[a]蒽)诱导的雌激素依赖性乳腺肿瘤的雌性Sprague-Dawley大鼠中: - 口服Anastrozole (ZD1033)(0.1 mg/kg/天或1 mg/kg/天)28天,肿瘤体积分别减少42% ± 4%(0.1 mg/kg)和65% ± 5%(1 mg/kg),肿瘤重量分别减少38% ± 3%(0.1 mg/kg)和62% ± 4%(1 mg/kg)[1] - 血清雌二醇水平较对照组分别降低80% ± 5%(0.1 mg/kg)和93% ± 4%(1 mg/kg)[1] 2. 芳香化酶抑制剂耐药模型疗效:在携带Anastrozole (ZD1033)耐药MCF-7移植瘤的裸鼠中: - 单独腹腔注射Anastrozole (ZD1033)(5 mg/kg,每周2次)对肿瘤生长无显著影响(21天内肿瘤体积增加25% ± 3%)。 - 与MK-2206(10 mg/kg,每周2次,腹腔注射)联合处理,肿瘤体积减少40% ± 4%,肿瘤组织中p-Akt表达降低65% ± 5%(免疫组化检测)[2] |
| 酶活实验 |
体外芳香酶抑制试验[1]
采用人胎盘微粒体和以睾酮(0.5 μM)为底物的Thompson和Siiteri法测定芳香酶抑制作用。11-羟化酶的抑制作用是通过使用新鲜制备的豚鼠、狗和牛肾上腺线粒体测量[1,2,6,7- 3h]- 1-脱氧-皮质醇向皮质醇的转化来确定的。反应产物提取成氯仿,用薄层色谱法分离[1]。 1. 微粒体制备: - 人胎盘组织或大鼠卵巢组织在含10 mM Tris-HCl的0.25 M蔗糖缓冲液(pH 7.4)中匀浆;匀浆经9,000×g离心20分钟去除碎片,上清液经105,000×g离心60分钟获得微粒体沉淀;沉淀重悬于含1 mM EDTA的50 mM Tris-HCl缓冲液(pH 7.4)中 [1] 2. 芳香化酶活性检测: - 反应体系(200 μL)含微粒体(15 μg蛋白)、[³H]-雄烯二酮(0.5 μM,底物)、NADPH(1 mM,辅酶)及不同浓度的Anastrozole (ZD1033)(0.01–100 nM),37°C孵育45分钟。 - 加入200 μL 1 M NaOH终止反应,10分钟后加入400 μL氯仿提取未反应底物;收集水相(含雌激素合成产物氚水),液体闪烁计数器检测放射性 [1] 3. 数据分析:采用米氏方程和非线性回归计算Ki值;通过对比实验组与对照组(无Anastrozole)的放射性强度确定抑制率 [1] |
| 细胞实验 |
Establishment of resistant cell lines and culture conditions/抗性细胞系的建立及培养条件[2]
从稳定转染了人芳香化酶基因(MCF-7aro)的ER+ mcf -7来源的乳腺癌细胞系22中,通过将这些MCF-7aro细胞暴露在不含酚红的Dulbecco's Modified Eagle培养基中,在20周内增加阿那曲唑的浓度(1,3和5µM),并添加3%类固醇缺失,葡聚糖包被和炭处理的胎牛血清(DCC培养基),其中含有25 nM 4-雄烯二酮(AD)。每次实验前,细胞在DCC培养基中清洗4天。每2天更换一次介质和治疗。[2] 细胞毒性试验[2]< br > 在96孔板中,每孔共104个细胞,并用AD联合阿那曲唑、来曲唑、4-羟基他莫昔芬(OH-Tam)、氟维司汀(ICI 182780)、MK-2206、雷帕霉素 或联合处理。细胞活力评估如前所述。 1. 乳腺癌细胞增殖实验(MTT法): - 敏感细胞:MCF-7细胞接种于96孔板(4×10³细胞/孔),用含5%活性炭剥离胎牛血清(CS-FBS)的无酚红RPMI 1640培养基培养24小时;细胞用Anastrozole (ZD1033)(0.1–100 nM)处理72小时;每孔加入20 μL MTT溶液(5 mg/mL)孵育4小时,DMSO溶解甲臜后检测570 nm吸光度 [1] - 耐药细胞:Anastrozole (ZD1033)耐药MCF-7细胞(敏感细胞经10 nM Anastrozole培养6个月构建)用Anastrozole(10–100 nM)单独或与MK-2206(1 μM)联合处理72小时,采用相同MTT法检测增殖 [2] 2. 信号通路Western blot检测: - 细胞用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解;30 μg蛋白裂解液经10% SDS-PAGE分离后转移至PVDF膜;膜与抗p-Akt(Ser473)、Akt、p-mTOR(Ser2448)、mTOR或β-肌动蛋白(内参)一抗孵育,再与二抗孵育;化学发光显影,密度分析法定量条带 [2] 3. 克隆形成实验: - 耐药MCF-7细胞(2×10³细胞/孔)接种于6孔板,用Anastrozole (ZD1033)(50 nM)单独或与MK-2206(1 μM)处理14天;甲醇固定克隆,结晶紫染色后计数;克隆形成率=(实验组克隆数/对照组克隆数)×100% [2] |
| 动物实验 |
0.1 mg/kg; oral Rats Aromatase inhibition. Groups of at least eight adult female rats (Alpk:AP~SD; Wistar derived), housed in controlled lighting (on 06.00-20.00 h) and temperature (24 + 2°C) and undergoing 4-day oestrous cycles, were treated p.o. with a single dose of anastrozole (0.01-0.1 mg/kg), fadrozole (0.01-0.1 mg/kg) or AG (5-20 mg/kg) on day 2 at 16.00 h or day 3 at 12.00 h. The presence or absence of eggs in the oviducts on day 1 of the next cycle was then determined. Ovulation was considered blocked when no eggs were found. Groups of eight immature (22-day-old) female rats were given AD (30 mg/kg) in arachis oil s.c. daily for 3 days with or without various doses of anastrozole p.o. On day 4 the uteri were dissected, blotted and weighed. Two groups of six mature male pigtailed monkeys (M. nernestrina) (body weights 11-21 kg) were used to compare effects of six dose levels (0.003, 0.01, 0.03, 0.1, 0.3 and 1.0 mg/kg) of anastrozole and fadrozole on plasma hormone concentrations. The drugs (in weights tailored to the individuals' body weights) were incorporated into peppermint candy and self administered twice daily (09.00 h and 16.00 h) by the monkeys. Each monkey was carefully observed for compliance; all doses were ingested. Blood samples were collected under ketamine sedation before the start of the study, after 7 days of treatment with the dosing vehicle (candy) and on the seventh day of treatment at each dose level. Blood collections were made at about the same time of day (15.00 h) on each occasion. Plasma was separated and stored at -20°C for hormone measurements (oestradiol, testosterone, cortisol and DOC). [1]
Adrenal function. Effects of anastrozole, metyrapone, AG and fadrozole on adrenal weights were determined in male rats (150-180 g) treated for 7 days. Effects (adrenal weight and histology) of anastrozole in five male and five female rats treated for 14 days and in three male and three female Alderley Park beagle dogs treated for 21 days were also determined; plasma K ÷ was also monitored in the dogs. Effects on aldosterone secretion were determined in groups of six male rats. Blood samples were collected from the abdominal aorta under halothane anaesthesia 2 h after an oral dose of anastrozole (5-20 mg/kg) or fadrozole (0.1-5 mg/kg) and heparinized plasma separated and stored at -20°C for aldosterone assays. Induced mineralocorticoid activity, a marker of elevated adrenal DOC secretion, was determined in groups of five male rats. These were given a single oral or s.c. dose of vehicle or anastrozole (5-10 mg/kg) or fadrozole (1-5 mg/kg) and, 1 h later, 2.5 ml of physiological saline s.c. Pooled urine from each group was collected during the next 5 h and Na ÷ and K ÷ concentrations were measured by flame ionization photometry. From the latter values, log10 (10 [Na÷]/ [K÷]) was calculated for each group; this compensates for differences in urine volumes and, for the saline load administered, the value of this function for control rats is approximately unity. [1] Steroid hormone activities Oestrogenic/anti-oestrogenic activity was assessed in a standard 3-day uterotrophic assay in immature (22-day-old) female rats (eight per group), with oestradiol benzoate (0.5 pg/rat/day s.c.) as standard. Androgenic/anti-androgenic activity was assessed by measuring ventral prostate and seminal vesicle weights in immature (22-day-old) male rats (eight per group) after 7 days of treatment, with testosterone propionate (2.5 mg/kg/day s.c.) as ,~tandard. Progestogenic activity was assessed by the capacity to maintain pregnancy in rats (groups of five) ovariectomized on day 9 of pregnancy (day 1 = sperm-positive smear) and given a maintenance dose of oestradiol (0.1 #g/rat) s.c. daily. Treatment was given on days 9-15 inclusive, and the uterine contents inspected post-mortem on day 16. Glucocorticoid/antiglucocorticoid activity was assessed by measuring thymus weights in immature female rats (groups of six) after 4 days of treatment, with dexamethazone (5 pg/rat/day i.p.) as standard. [1] 1. DMBA-induced rat mammary tumor model: - Model establishment: Female Sprague-Dawley rats (50 days old) were gavaged with DMBA (20 mg/kg, dissolved in sesame oil) to induce estrogen-dependent mammary tumors. Tumors were allowed to grow to 100–150 mm³ before treatment [1] - Grouping and treatment: Rats were randomly divided into 3 groups (n=8/group): - Control group: Oral gavage of 0.5% carboxymethyl cellulose (CMC) once daily for 28 days. - Low-dose group: Oral gavage of Anastrozole (ZD1033) (0.1 mg/kg/day, dissolved in 0.5% CMC) once daily for 28 days. - High-dose group: Oral gavage of Anastrozole (ZD1033) (1 mg/kg/day, dissolved in 0.5% CMC) once daily for 28 days [1] - Detection: Tumor volume was measured twice weekly (volume = length × width² / 2). After 28 days, rats were euthanized; tumors were weighed, and serum was collected to measure estradiol levels by radioimmunoassay [1] 2. Nude mouse xenograft model (resistant cells): - Model establishment: Female nude mice (6–8 weeks old) were subcutaneously injected with Anastrozole (ZD1033)-resistant MCF-7 cells (5×10⁶ cells/mouse, suspended in Matrigel) into the right flank. Tumors were allowed to grow to 80–100 mm³ before treatment [2] - Grouping and treatment: Mice were randomly divided into 3 groups (n=6/group): - Control group: Intraperitoneal injection of normal saline twice weekly for 21 days. - Anastrozole group: Intraperitoneal injection of Anastrozole (ZD1033) (5 mg/kg) twice weekly for 21 days. - Combination group: Intraperitoneal injection of Anastrozole (ZD1033) (5 mg/kg) + MK-2206 (10 mg/kg) twice weekly for 21 days [2] - Detection: Tumor volume was measured every 3 days. After 21 days, mice were euthanized; tumor tissues were collected for immunohistochemical detection of p-Akt [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Anastrozole is rapidly absorbed and Tmax is typically reached within 2 hours of dosing under fasted conditions. Coadministration with food reduces the rate but not the overall extent of absorption - mean Cmax decreased by 16% and the median Tmax was extended to 5 hours when anastrozole was administered 30 minutes after ingestion of food, though this relatively minor alteration in absorption kinetics is not expected to result in clinically significant effects. Hepatic metabolism accounts for approximately 85% of anastrozole elimination. Approximately 10% of the administered dosage is eliminated unchanged in the urine. The volume of distribution of anastrozole into brain tissue in mice is 3.19 mL/g. Distribution into the CNS is limited due to the activity of P-gp efflux pumps at the blood brain barrier, of which anastrozole is a substrate. Anastrozole's clearance is mainly via hepatic metabolism and can therefore be altered in patients with hepatic impairment - patients with stable hepatic cirrhosis exhibit an apparent oral clearance approximately 30% lower compared with patients with normal liver function. Conversely, renal impairment has a negligible effect on total drug clearance as the renal route is a relatively minor clearance pathway for anastrozole. In volunteers with severe renal impairment, renal clearance was reduced by 50% while total clearance was only reduced by approximately 10%. Anastrozole is well absorbed into systemic circulation following oral administration. Plasma concentrations approach steady-state at about 7 days of once-daily dosing, and steady-state concentrations are approximately 3-4 times higher than concentrations achieved after a single dose of the drug. Food does not affect the extent of oral absorption of anastrozole. Within the therapeutic plasma concentration range, anastrozole is 40% bound to plasma proteins. Steady-state minimum plasma concentrations averaged 25.7 and 30.4 ng/mL, respectively, in white and Japanese postmenopausal women receiving anastrozole 1 mg daily for 16 days; serum estradiol and estrone sulfate concentrations were similar between the groups. It is not known whether anastrozole is distributed into milk in humans. For more Absorption, Distribution and Excretion (Complete) data for ANASTROZOLE (10 total), please visit the HSDB record page. Metabolism / Metabolites Anastrozole is primarily metabolized in the liver via oxidation and glucuronidation to a number of inactive metabolites, including hydroxyanastrozole (both free and glucuronidated) and anastrozole glucuronide. Oxidation to hydroxyanastrozole is catalyzed predominantly by CYP3A4 (as well as CYP3A5 and CYP2C8, to a lesser extent) and the direct glucuronidation of anastrozole appears to be catalyzed mainly by UGT1A4. Anastrozole may also undergo N-dealkylation to form triazole and 3,5-Bis-(2-methylpropiononitrile)-benzoic acid. Labels for anastrozole state the main metabolite found in plasma following administration is triazole, but a recent pharmacokinetic study was unable to detect any products of N-dealkylation _in vitro_. Anastrozole is extensively metabolized in the liver. Metabolism of anastrozole occurs via N-dealkylation, hydroxylation, and glucuronidation. Three metabolites of anastrozole have been identified in human plasma and urine: triazole, a glucuronide conjugate of anastrozole, and a glucuronide conjugate of hydroxyanastrozole. Triazole, the major circulating metabolite of anastrozole, lacks pharmacologic activity, and the aromatase inhibiting activity of anastrozole results principally from the parent drug. In addition, there are several minor metabolites of anastrozole, accounting for less than 5% of an administered dose, which have not been identified. Hepatic. Metabolized mainly by N-dealkylation, hydroxylation, and glucuronidation to inactive metabolites. Primary metabolite is an inactive triazole. Route of Elimination: Hepatic metabolism accounts for approximately 85% of anastrozole elimination. Renal elimination accounts for approximately 10% of total clearance. Half Life: 50 hours Biological Half-Life The elimination half-life of anastrozole is approximately 50 hours. Following oral administration of anastrozole in postmenopausal women, a mean terminal elimination half-life of approximately 50 hours has been reported. Absorption: The oral bioavailability of Anastrozole (ZD1033) in rats was approximately 70%, and food intake did not affect its absorption. Peak plasma concentration (Cmax) of 120 ± 15 ng/mL was reached 2 hours after oral administration of 1 mg/kg [1] - Distribution: The volume of distribution (Vd) of Anastrozole (ZD1033) in rats was 1.2 ± 0.1 L/kg. It distributed widely in tissues, with tumor tissue concentrations 2.5-fold higher than plasma concentrations at 4 hours post-dosing [1] - Elimination: The elimination half-life (t1/2) of Anastrozole (ZD1033) in rats was 15 ± 2 hours. Approximately 60% of the dose was excreted in feces and 30% in urine within 72 hours, mainly as unchanged drug [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Anastrozole selectively inhibits aromatase. The principal source of circulating estrogen (primarily estradiol) is conversion of adrenally-generated androstenedione to estrone by aromatase in peripheral tissues. Therefore, aromatase inhibition leads to a decrease in serum and tumor concentration of estrogen, leading to a decreased tumor mass or delayed progression of tumor growth in some women. Anastrozole has no detectable effect on synthesis of adrenal corticosteroids, aldosterone, and thyroid hormone. Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (L97) Hepatotoxicity Serum enzymes are reported to be elevated in 2% to 4% of women treated with anastrozole, but these elevations are usually mild, asymptomatic and self-limited, rarely requiring dose modification. There have been rare instances of clinically apparent liver injury associated with anastrozole therapy, typically arising within 1 to 4 months of starting treatment and having variable presentations but generally with a hepatocellular or mixed serum enzyme pattern (Case 1). Too few instances have been described in the literature to provide specific characteristics or clinical phenotype. Immunoallergic features (fever, rash, eosinophilia) were not mentioned in published cases, but low levels of autoantibodies were sometimes found. Recovery is usually rapid once anastrozole is stopped. There have been no cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome attributed to anastrozole use. Unlike tamoxifen, anastrozole has not been associated with development of fatty liver disease, although some degree of steatosis and steatohepatitis have been mentioned in descriptions of liver biopsies from acute cases. According to the product label, anastrozole has been linked to cases of hypersensitivity reactions and Stevens-Johnson syndrome as well as cases of hepatitis with jaundice. Likelihood score: C (probable cause of clinically apparent liver injury). Protein Binding Anastrozole is 40% protein bound in plasma and appears to be independent of plasma concentration. Toxicity Data In rats, lethality is greater than 100 mg/kg. Interactions Administration of a single 30 mg/kg or multiple 10 mg/kg doses of anastrozole to healthy subjects had no effect on the clearance of antipyrine or urinary recovery of antipyrine metabolites. Based on these in vitro and in vivo results, it is unlikely that co-administration of anastrozole 1 mg with other drugs will result in clinically significant inhibition of cytochrome P450 mediated metabolism. In a study conducted in 16 male volunteers, anastrozole did not alter the pharmacokinetics as measured by Cmax and AUC, and anticoagulant activity as measured by prothrombin time, activated partial thromboplastine time, and thrombin time of both R- and S-warfarin. Co-administration of anastrozole and tamoxifen in breast cancer patients reduced anastrozole plasma concentration by 27% compared to those achieved with anastrozole alone; however, the coadministration did not affect the pharmacokinetics of tamoxifen or N-desmethyltamoxifen. Acute toxicity: The median lethal dose (LD50) of Anastrozole (ZD1033) was >2000 mg/kg in mice (oral) and >1500 mg/kg in rats (oral) [1] - Chronic toxicity: In a 3-month oral toxicity study in rats (doses of 0.1, 1, 10 mg/kg/day), no significant changes in liver function (ALT/AST) or kidney function (creatinine/BUN) were observed. Uterine weight was reduced by 35% ± 4% in the 10 mg/kg group (due to estrogen deprivation) [1] - Plasma protein binding: Anastrozole (ZD1033) had a plasma protein binding rate of 90% ± 2% in human plasma and 88% ± 3% in rat plasma [1] |
| 参考文献 |
[1]. Dukes M, et al. The preclinical pharmacology of "Arimidex" (anastrozole; ZD1033)--a potent, selective aromatase inhibitor. J Steroid Biochem Mol Biol. 1996 Jul;58(4):439-45.
[2]. Molecular characterization of anastrozole resistance in breast cancer: Pivotal role of the Akt/mTOR pathway in the emergence of de novo or acquired resistance and importance of combining the allosteric Akt inhibitor MK-2206 with an aromatase inhibitor. Int J Cancer. 2013 Oct 1;133(7):1589-602. |
| 其他信息 |
Therapeutic Uses
Antineoplastic Anastrozole is indicated for the first-line treatment of postmenopausal woman with hormone receptor positive or hormone receptor unknown locally advanced or metastatic breast cancer. It is also indicated for treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. /Included in US product label/ Anastrozole is an option for the neoadjuvant treatment of hormone receptorpositive, locally advanced breast cancer in postmenopausal women. Two phase 2, randomized, double-blind clinical trials found anastrozole to be at least as effective as tamoxifen in response rates and rates of improved surgery. A phase 2, unpublished abstract reported no differences between neoadjuvant anastrozole and chemotherapy (doxorubicin and paclitaxel) in response rates, number of patients qualifying for breast-conserving surgery, and 3-year disease-free survival. An international expert panel recommends neoadjuvant endocrine therapy in postmenopausal women who would benefit from preoperative chemotherapy but are ineligible to receive it. Anastrozole was well-tolerated. /Not included in US product label/ Anastrozole is not recommended for use in premenopausal women. Safety and efficacy have not been established. /Included in US product label/ For more Therapeutic Uses (Complete) data for ANASTROZOLE (8 total), please visit the HSDB record page. Drug Warnings Among patients receiving adjuvant therapy, venous thromboembolic events occurred less frequently in patients receiving anastrozole than in those receiving tamoxifen (2 versus 4%); this included deep venous thrombosis (1 versus 2%). Ischemic cerebrovascular events also occurred less frequently in patients receiving anastrozole compared with those receiving tamoxifen (1 versus 2%). Ischemic cardiovascular disease was reported in 3% of such patients receiving anastrozole. Although angina pectoris was reported more frequently in patients receiving adjuvant therapy with anastrozole than in those receiving tamoxifen (about 2 versus 1%), the incidence of myocardial infarction was similar (0.8%). Among patients receiving anastrozole as first-line therapy, thromboembolic disease was reported in 18 patients (4%), with 5 patients experiencing venous thrombosis (including pulmonary embolus, thrombophlebitis, and retinal vein thrombosis) and 13 patients experiencing coronary and/or cerebral thrombosis (including myocardial infarction, myocardial ischemia, angina pectoris, cerebrovascular accident, cerebral ischemia, and cerebral infarct). Despite its lack of estrogenic activity, there was no evidence of an increased incidence of myocardial infarction in patients receiving anastrozole compared with those receiving tamoxifen. Among patients receiving anastrozole as second-line therapy, thromboembolic disease was reported in 3%, and thrombophlebitis occurred in 2-5%. Among patients receiving adjuvant therapy, hot flushes (flashes) occurred less frequently in patients receiving anastrozole than in those receiving tamoxifen (35 versus 40%). Among patients receiving anastrozole as first-line or second-line therapy, hot flushes occurred in 26 or 13%, respectively. For more Drug Warnings (Complete) data for ANASTROZOLE (35 total), please visit the HSDB record page. Pharmacodynamics Anastrozole prevents the conversion of adrenal androgens (e.g. [testosterone]) to estrogen in peripheral and tumour tissues. As the growth of many breast cancers is stimulated and/or maintained by the presence of estrogen, anastrozole helps to treat these cancers by decreasing the levels of circulating estrogens. Anastrozole has a relatively long duration of action allowing for once daily dosing - serum estradiol is reduced by approximately 70% within 24 hours of beginning therapy with 1mg once daily, and levels remain suppressed for up to 6 days following cessation of therapy. The incidence of ischemic cardiovascular events was increased during anastrozole therapy and patients with pre-existing ischemic heart disease should consider the risks and benefits of anastrozole before beginning therapy. Anastrozole has also been reported to decrease spine and hip bone mineral density (BMD), so consideration should be given to monitoring of BMD in patients receiving long-term therapy. 1. Anastrozole (ZD1033) is a potent, selective, non-steroidal aromatase inhibitor (AI) that specifically blocks estrogen biosynthesis by inhibiting aromatase, the key enzyme converting androgens to estrogens. It is more selective for aromatase than earlier AIs (e.g., aminoglutethimide) [1] 2. In estrogen-dependent breast cancer, Anastrozole (ZD1033) exerts antitumor effects by reducing estrogen levels, thereby inhibiting ERα-mediated cell proliferation. However, long-term use can induce acquired resistance via activation of the Akt/mTOR pathway [2] 3. Combination of Anastrozole (ZD1033) with Akt inhibitors (e.g., MK-2206) can reverse acquired resistance by suppressing the Akt/mTOR pathway, providing a potential therapeutic strategy for resistant breast cancer [2] |
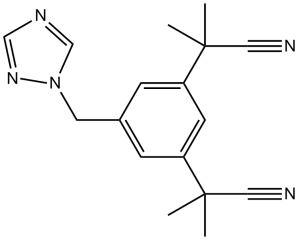
| 分子式 |
C17H19N5
|
|---|---|
| 分子量 |
293.3663
|
| 精确质量 |
293.164
|
| 元素分析 |
C, 69.60; H, 6.53; N, 23.87
|
| CAS号 |
120511-73-1
|
| 相关CAS号 |
Anastrozole-d12;120512-32-5
|
| PubChem CID |
2187
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
469.7±55.0 °C at 760 mmHg
|
| 熔点 |
81-82°C
|
| 闪点 |
237.9±31.5 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.580
|
| LogP |
0.97
|
| tPSA |
78.29
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
456
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1(C([H])=NC([H])=N1)C([H])([H])C1C([H])=C(C([H])=C(C=1[H])C(C#N)(C([H])([H])[H])C([H])([H])[H])C(C#N)(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
YBBLVLTVTVSKRW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
|
| 化学名 |
2,2'-(5-((1H-1,2,4-triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile)
|
| 别名 |
ZD-1033; ZD1033; ZD 1033; CCRIS 9352; HSDB 7462; ICI D1033; Anastrozole (ANAS); 120511-73-1; Arimidex; anastrazole; Anastrozol; ZD1033; 2,2'-(5-((1H-1,2,4-triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile); Asiolex; Trade name: Arimidex.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4087 mL | 17.0433 mL | 34.0866 mL | |
| 5 mM | 0.6817 mL | 3.4087 mL | 6.8173 mL | |
| 10 mM | 0.3409 mL | 1.7043 mL | 3.4087 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。