| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Aromatase
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:氨基鲁米特在体外用人胎盘芳香酶测定时表现出芳香酶抑制作用,芳香酶是一种参与雄激素转化为雌激素的酶,也是乳腺癌内分泌治疗的重要靶点。 Aminogluthimide 以时间依赖性方式抑制绵羊肾上腺皮质细胞中 ACTH 受体 (ACTH-R) mRNA 的表达。与对照细胞相比,氨基鲁米特以剂量依赖性方式显着抑制类固醇分泌和基线 ACTH-R mRNA 表达(300 μM AG,5±1%;30 μM AG,64±1%;3 μM AG,108±19%) , 100±11%) 通过影响基因表达或通过影响 RNA 稳定性来减少转录物积累,在人 NCI-h295 肾上腺皮质癌细胞系中,该细胞系表达功能性 ACTH 受体并产生糖皮质激素、盐皮质激素和雄激素途径的类固醇。 Aminogluthimide 以剂量依赖性方式抑制芳香酶,在 6 个乳腺肿瘤匀浆中的 IC50 为 13 μM,胎盘芳香酶的 IC50 为 6 μM,下丘脑芳香酶的 IC50 为 8 μM。激酶测定:微粒体蛋白 (30 μg)、[1β-3H]雄烯二酮 (6.6 × 105 dpm) 和 NADPH (270 μM) 用于浓度响应实验,孵育时间为 20 分钟。氨基鲁米特最初在 10 μM 和 100 μM 浓度下进行测试,然后使用至少 8 个浓度(从 0.01 μM 到 160 μM)进行完整的浓度-反应研究。对于初始速度研究,[1β-3H]雄烯二酮的浓度在 7.5 至 100 nM 之间变化,孵育时间设置为 5 分钟。通过液体闪烁计数对氚化底物[1β-3H]雄烯二酮转化为雌酮过程中形成的氚化水进行定量。每个测定重复进行三次,并通过非线性回归分析处理结果,从而确定半数最大抑制浓度(IC50)。细胞测定:NCI-h295 肿瘤细胞系维持在补充有转铁蛋白 (0.1 mg/mL)、胰岛素 (5 μg/mL)、硒 (5.2 μg/mL) 和 2% FCS 的 RPMI 1640 培养基中。将细胞与氨基鲁米特(3、30、300 μM)一起孵育 48 小时。然后通过台盼蓝染色检查细胞的细胞活力,并用库尔特计数器进行计数。为了评估 ACTH-R mRNA,收获细胞,提取总 RNA,进行电泳、印迹并与人 ACTH-R cDNA 探针杂交。
|
||
| 体内研究 (In Vivo) |
服用氨鲁米特1至2周后,氨鲁米特加速其自身代谢,从基础值2.6±0.3(SE)升/24小时增加至5.3±1.4升/24小时,并显着加速合成糖皮质激素和地塞米松的代谢,从施用氨基鲁米特2周后,基础值为145±26.6升/24小时至568±127升/24小时(p < 0.02)。 Aminogluthimide (150 mg/kg) 消除鸟氨酸脱羧酶 (ODC) 的诱导,几乎耗尽成年雌性小鼠卵巢和未成熟雄性小鼠睾丸中由人绒毛膜促性腺激素 (hCG) 引起的性腺和血浆黄体酮或睾酮,这与 cAMP 依赖性蛋白激酶的抑制 (IC50 287 μM) 有关,而不是与类固醇生成途径的阻断有关。
使用类固醇生物合成抑制剂抑制雌激素的产生是治疗激素依赖性癌症妇女的合理策略。氨基鲁米特作为细胞色素P-450介导的类固醇羟基化抑制剂的临床可用性促使人们研究该药物的精确药理学和生化作用。药代动力学研究表明,氨基鲁米特会改变其自身的代谢清除率以及合成糖皮质激素地塞米松的代谢清除速率。其他类固醇如氢化可的松、醋酸甲羟孕酮、雄烯二酮和雌酮的代谢清除率不会因氨基鲁米特而改变。这些发现导致了一种实用的方案的开发,即逐步增加氨基鲁米特剂量联合氢化可的松治疗乳腺癌患者。进一步的研究集中在氨基鲁米特抑制雌激素的生化机制上。在体内,同位素动力学数据表明,氨基鲁米特对绝经后妇女的外周芳香化酶抑制率为95%至98%。体外实验表明,氨基鲁米特也能有效直接阻断人乳腺肿瘤中的芳香化酶。就相对效力而言,氨基鲁米特是一种效力比睾酮强10倍的芳香化酶抑制剂,但效力不如4-羟基雄烯二酮和几种溴化雄烯二酮类衍生物。综上所述,这些研究表明,氨鲁米特在乳腺癌女性的三个部位阻断了雌激素的产生:肾上腺皮质、含有芳香化酶的腺外周组织和乳腺癌组织本身[1]。 氨基谷草胺是一种类固醇生成抑制剂,可抑制胆固醇侧链切割酶(CYP11A1),该酶在线粒体中将胆固醇转化为孕烯醇酮。我们研究了给予AG 5天对小鼠的组织病理学变化。AG处理的小鼠束状带细胞中发现了各种大小的细胞质空泡和单细胞坏死。在免疫组织化学染色中,一些液泡对嗜脂蛋白呈阳性,而另一些液泡对溶酶体相关膜蛋白-2呈阳性,表明它们分别是增大的脂滴和溶酶体。电子显微镜显示,束状带细胞中溶酶体增大,含有受损的线粒体和板层体,它们被认为反映了细胞内蛋白质降解过程、自噬和亲脂性。从这些结果中,我们发现AG在束状带细胞中诱导了过度的脂质积累和线粒体损伤,导致小鼠溶酶体加速降解[4]。 |
||
| 酶活实验 |
激酶活性测定:微粒体蛋白(30μg)、[1β-3H]雄烯二酮(6.6×105 dpm)和NADPH(270μM)用于浓度反应实验,孵育时间为20分钟。首先,在10μM和100μM的浓度下对氨基谷草胺进行测试,然后进行至少8个浓度范围在0.01μM至160μM的完整浓度-反应研究。对于初始速度研究,[1β-3H]雄烯二酮的浓度在7.5至100 nM之间变化,孵育时间设置为5分钟。通过液体闪烁计数对氚化底物[1β-3H]雄烯二酮转化为雌酮过程中形成的氚化水进行定量。每次测定进行三次,两次,结果通过非线性回归分析进行处理,从而确定半最大抑制浓度(IC50)。
|
||
| 细胞实验 |
离体皮质培养和细胞死亡试验[3]
如前所述(Shirakawa等人,2002),从总共16只母鼠的胎仔Wistar大鼠(妊娠17-19天)的大脑皮层制备分离皮层神经元的原代培养物。将从整个大脑皮层机械分离的单细胞以4.5×105个细胞cm−2的密度接种到涂有聚乙烯亚胺的48孔板上(用于细胞死亡分析)或涂有聚乙烯胺的玻璃盖玻片上(用于Ca2+测量)。细胞在Eagle的补充了谷氨酰胺(2 mM)、葡萄糖(总共11 mM)、NaHCO3(24 mM)、HEPES(10 mM)和10%热灭活胎牛血清(1-7 DIV)或10%热灭活马血清(8-12 DIV)的最低必需培养基中,在加湿的5%CO2气氛中保持在37°C。在6 DIV时加入10μM阿糖胞苷可抑制非神经元细胞的增殖。 在11 DIV时,将谷氨酸以300μM的终浓度加入培养基中24小时,并通过上述切片培养实验中的LDH释放试验评估细胞死亡,但将15μl培养基与30μl LDH底物混合物和60μl 10mM磷酸缓冲盐水混合。使用用10mM谷氨酸处理24小时的培养物来确定每组实验中的标准损伤程度。吸光度值以接受标准损伤的培养物中的吸光度为100%进行归一化。 我们还通过3-(4,5-二甲基-2-噻唑基)-2,5-二苯基溴化四唑(MTT)法评估了细胞存活率。将培养的细胞在含有0.5 mg ml-1 MTT的Eagle培养基中孵育2小时,然后用异丙醇溶解,测量595 nm处的吸光度。通过将对照培养物的值设置为100%,将接受标准损伤(10mM谷氨酸盐24小时)的培养物值设置为0%,将存活率表示为对照的%。 细胞内Ca2+浓度的测量[3] 谷氨酸诱导的细胞内Ca2+浓度([Ca2+]i)的增加用Ca2+敏感的荧光染料fura-2乙酰氧基甲酯和荧光成像系统进行了估算。在聚乙烯亚胺包被的玻璃盖玻片上培养的11-12DIV分离的皮质神经元在Krebs-Ringer缓冲液(137mM NaCl,5mM KCl,1mM MgCl2,1.5mM氯化钙,10mM HEPES,25 mM葡萄糖,pH 7.4),含有5μM呋喃-2-乙酰氧基甲酯和0.01%乳粉EL,在37°C下放置30分钟。在不含呋喃-2-的克雷布斯-林格缓冲液中插管至少30分钟后,将盖玻片转移到倒置荧光显微镜载物台上的记录室中。在室温下每3秒记录一次通过在340和380nm波长下激发获得的Fura-2荧光。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and completely absorbed from gastrointestinal tract. The bioavailability of tablets is equivalent to equal doses given as a solution. After ingestion of a single oral dose, 34%-54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative. Cytadren is rapidly and completely absorbed after oral administration. In 6 healthy male volunteers, maximum plasma levels of Cytadren averaged 5.9 ug/mL at a medium of 1.5 hours after ingestion of 250 mg tablets. The bioavailability of tablets is equivalent to equal doses given as a solution. Aminoglutethimide crosses the placenta ... It is not known weather aminoglutethimide is distributed into breast milk. After ingestion of a single oral dose, 34% to 54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative. For more Absorption, Distribution and Excretion (Complete) data for AMINOGLUTETHIMIDE (7 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. 34-54% of the administered dose is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as an N-acetyl derivative. Hepatic; the major metabolite is N-acetylaminoglutethimide; there may be genetic variation among individuals in the rate of acetylation. Four ... metabolites of aminoglutethimide have been identified in the urine of patients being treated chronically with the drug. These were products of hydroxylation of the 3-ethylpiperidine-2,6-dione residue, namely 3-(4-aminophenyl)-3-ethyl-5-hydroxypiperidine-2,6-dione and its acetylamino analog, 3-(4-aminophenyl)-3-(1-hydroxyethyl)piperidine-2,6-dione, and 3-(4-aminophenyl)-3-(2-carboxamidoethyl)tetrahydrofuran-2-one, the lactone formed by rearrangement of 3-(4-aminophenyl)-3-(2-hydroxyethyl)piperidine-2,6-dione. ... These new metabolites were minor constituents compared with aminoglutethimide and with the previously identified major metabolites 3-(4-acetylaminophenyl)-3-ethylpiperidine-2,6-dione and 3-(4-hydroxylaminophenyl)-3-ethylpiperidine-2,6-dione. There were marked species differences between rat and human inasmuch as almost all the metabolites in the urine of the rat were N-acetylated whereas most of the human metabolites were not. However, 5-hydroxylation of the piperidinedione residue was stereoselective in the same sense in both species, the cis isomer being formed exclusively. Synthetic cis-3-(4-aminophenyl)-3-ethyl-5-hydroxypiperidine-2,6-dione did not inhibit the activity of the target enzyme systems desmolase and aromatase in vitro, and therefore, like other metabolites so far described, is an inactivation product of the drug. Hydroxylaminoglutethimide (3-ethyl-3-(4-hydroxylaminophenyl)-2,6-piperidinedione) has been identified as a novel metabolite of aminoglutethimide (3-(4-aminophenyl)-3-ethyl-2,6-piperidinedione) in the urine of patients treated chronically with this drug. The metabolite was isolated by reverse-phase thin-layer chromatography, and characterized by comparison of its mass spectrum and chromatographic properties with those of the synthetic compound. Hydroxylaminoglutethimide is unstable; it is readily oxidized to nitrosoglutethimide and disproportionates in the mass spectrometer into this compound and aminoglutethimide. In none of four patients studied was the metabolite detected in the urine after the first dose of the drug. In one patient it appeared after the second dose and in two more within seven to eight days suggesting that its formation is drug-induced, and that it may be the metabolite responsible for the diminished half-life of aminoglutethimide during chronic therapy. The profile of metabolites from one patient, examined by high-performance liquid chromatography after the first dose and again after six weeks of therapy afforded evidence that the formation of hydroxylaminoglutethimide was at the expense of a major metabolite N-acetylaminoglutethimide. Hydroxylaminoglutethimide [3-ethyl-3-(4-hydroxylaminophenyl)piperidine-2,6-dione] (HxAG), aminoglutethimide [3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione] (AG) and N-acetyl-aminoglutethimide (N-AcAG) have been quantified by high performance liquid chromatography using m-aminoglutethimide (metaAG) as the internal standard in serial 24 hr urine collections from a patient on chronic AG therapy without steroid supplementation. HxAG is the product of a major AG-induced metabolic pathway since the ratio [HxAG]/[AG] rises with time. In contrast the ratio [N-AcAG]/[AG] decreases with time. A rapid, simple colorimetric assay has been used to quantify HxAG in urine from both male and female patients receiving a range of doses of AG and to show that induced metabolism is a general phenomenon even at low doses (125 mg twice daily). Extensive metabolism occurred in all species, with N-acetylaminoglutethimide being the major metabolite except for dog and man. In the latter two species unchanged drug was the main product excreted. A metabolite, 3-(4-acetamidophenyl)-3-(2-carboxamidoethyl)tetrahydrofuran-2-one, not previously found in human urine, was identified. Chronic administration of aminoglutethimide to rats produced no detectable change in the excretory or metabolite patterns of the drug. However chronic administration of phenobarbitone decreased the urinary excretion of (14)C over a 72 hr period. Residual (72 hr) tissue levels of (14)C were less than 1 microgram equivalent of (14)C-aminoglutethimide/g tissue in the rat, guinea-pig and rabbit. Dog tissues retained a considerable quantity of (14)C at this time. Hepatic. 34-54% of the administered dose is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as an N-acetyl derivative. Route of Elimination: After ingestion of a single oral dose, 34%-54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative. Half Life: 12.5 ± 1.6 hours Biological Half-Life 12.5 ± 1.6 hours 12.5 hours; reduced to 7 hours after prolonged (2 to 32 weeks) treatment because aminoglutethimide induces hepatic enzymes and accelerates its own metabolism. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Aminoglutethimide reduces the production of D5-pregnenolone and blocks several other steps in steroid synthesis, including the C-11, C-18, and C-21 hydroxylations and the hydroxylations required for the aromatization of androgens to estrogens, mediated through the binding of aminoglutethimide to cytochrome P-450 complexes. Specifically, the drug binds to and inhibits aromatase which is essential for the generation of estrogens from androstenedione and testosterone. A decrease in adrenal secretion of cortisol is followed by an increased secretion of pituitary adrenocorticotropic hormone (ACTH), which will overcome the blockade of adrenocortical steroid synthesis by aminoglutethimide. The compensatory increase in ACTH secretion can be suppressed by the simultaneous administration of hydrocortisone. Since aminoglutethimide increases the rate of metabolism of dexamethasone but not that of hydrocortisone, the latter is preferred as the adrenal glucocorticoid replacement. Although aminoglutethimide inhibits the synthesis of thyroxine by the thyroid gland, the compensatory increase in thyroid-stimulating hormone (TSH) is frequently of sufficient magnitude to overcome the inhibition of thyroid synthesis due to aminoglutethimide. In spite of an increase in TSH, aminoglutethimide has not been associated with increased prolactin secretion. Protein Binding 21-25% Toxicity Data Oral LD50s (mg/kg): rats, 1800; dogs, >100. Intravenous LD50s (mg/kg): rats, 156; dogs, >100. Interactions Aminoglutethimide may inhibit the adrenal response to ACTH; this may interfere with the therapeutic response to ACTH. /Upon concomitant administration of/ CNS depression-producing medications, additive CNS depression may occur. Hyponatremia may occur /with concomitant administration of/ diuretics. Cytadren accelerates the metabolism of dexamethasone... For more Interactions (Complete) data for AMINOGLUTETHIMIDE (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip 625 mg/kg |
||
| 参考文献 | |||
| 其他信息 |
Therapeutic Uses
Adrenocortical suppressant; antineoplastic Aminoglutethimide is indicated for temporary suppression of adrenal function in selected patients with Cushing's syndrome including that associated withadrenal carcinoma and etopic adrenocorticotropic hormone (ACTH)-producing tumors or adrenal hyperplasia. /Included in US product label/ Aminoglutethimide is indicated to produce a "pharmacologic adrenalectomy" in the treatment of post menopausal metastatic breast cancer, especially inoperable or recurrent breast cancer proven to be hormone dependent, but resistant to therapy with tamoxifen. /Included in US product label/ Aminoglutethimide is indicated for treatment of prostatic carcinoma unresponsive to hormonal or surgical therapy. /Included in US product label/ For more Therapeutic Uses (Complete) data for AMINOGLUTETHIMIDE (7 total), please visit the HSDB record page. Drug Warnings Cytadren may cause adrenocortical hypofunction, especially under conditions of stress, such as surgery, trauma, or acute illness. Patients should be carefully monitored and given hydrocortisone and mineralocorticoid supplements as indicated. Dexamethasone should not be used. Cytadren may also suppress aldosterone production by the adrenal cortex and may cause orthostatic or persistent hypotension. The blood pressure should be monitored in all patients at appropriate intervals. Patients should be advised of the possible occurrence of weakness and dizziness as symptoms of hypotension, and measures to be taken should they occur. Cytadren can cause fetal harm when administered to a pregnant woman. In the earlier experience with the drug in about 5000 patients, two cases of pseudohermaphroditism were reported in female infants whose mothers were treated with Cytadren ... If this drug must be used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus. Patients should be warned that drowsiness may occur and that they should not drive, operate potentially dangerous machinery, or engage in other activities that may become hazardous because of decreased alertness. For more Drug Warnings (Complete) data for AMINOGLUTETHIMIDE (19 total), please visit the HSDB record page. Pharmacodynamics Aminoglutethimide inhibits the enzymatic conversion of cholesterol to D5-pregnenolone, resulting in a decrease in the production of adrenal glucocorticoids, mineralocorticoids, estrogens, and androgens. Aminoglutethimide is a clinically available drug that suppresses steroid biosynthesis by inhibiting enzymes such as cytochrome P450scc and aromatase. Because several members of neurosteroids regulate glutamate receptors, we investigated the effect of aminoglutethimide on cell death induced by overactivation of glutamate receptors in CNS neurons. Long-term pretreatment of organotypic cerebrocortical slice cultures with aminoglutethimide (100–1000 μM) for 6 days or over resulted in concentration-dependent suppression of neuronal cell death induced by NMDA. Aminoglutethimide (1000 μM) also inhibited neurotoxicity of AMPA and kainate, but not of ionomycin or staurosporine. The protective effect of aminoglutethimide against NMDA cytotoxicity was not mimicked by other steroid synthesis inhibitors including trilostane and exemestane, and was not reversed by concurrent application of steroids such as pregnenolone, estrone, 17β-estradiol and estriol. In dissociated rat cerebrocortical cell cultures, long-term treatment with aminoglutethimide (10–1000 μM) attenuated NMDA receptor-mediated glutamate cytotoxicity but produced no significant effect on glutamate-induced increases in intracellular Ca2+. Brief as well as long-term pretreatment with aminoglutethimide (30–1000 μM) prevented NMDA receptor-dependent ischemic neuronal injury in organotypic cerebrocortical slice cultures, which was associated with suppression of glutamate release during the ischemic insult. These results indicate that aminoglutethimide, irrelevant to its actions on neurosteroid synthesis, protects CNS neurons from excitotoxic and ischemic injuries. Development of aminoglutethimide analogs possessing neuroprotective properties may be of therapeutic value.[3] Abstract Aromatase, an enzyme involved in the conversion of androgens into estrogens, is an important target for the endocrine treatment of breast cancer. Aromatase inhibition is usually achieved with steroids structurally related to the substrate of catalysis or, alternatively, with azole non-steroid compounds. Substituted androstenedione derivatives with Delta(1), Delta(6) and Delta(1,6) unsaturations and 6-alkyl/6-phenyl aliphatic substitutions, are among the most potent steroid aromatase inhibitors known to date. In this paper we have combined the common pharmacophoric and shape features of these molecules into a new pharmacophore model, useful for virtual screening of large compound databases. Small subsets of the best fitting anti-aromatase candidates were extracted from the NCI database and experimentally tested on an in vitro assay with human placental aromatase. New potent aromatase inhibitors were identified such as compounds 8 and 14. Considering the lack of a crystal structure for the aromatase enzyme, this ligand-based method is a valuable tool for the virtual screening of new aromatase inhibitors. [2] |
| 分子式 |
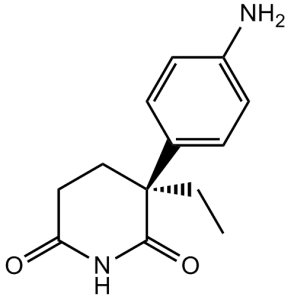
C13H16N2O2
|
|
|---|---|---|
| 分子量 |
232.28
|
|
| 精确质量 |
232.121
|
|
| 元素分析 |
C, 67.22; H, 6.94; N, 12.06; O, 13.78
|
|
| CAS号 |
125-84-8
|
|
| 相关CAS号 |
125-84-8; 23734-88-5 (phosphate); 57344-88-4 [(R)-(+)-Aminoglutethimide L-Tartrate]; 57288-03-6 (S-isomer); 57288-04-7 [S-(-)-Aminoglutethimide D-tartrate]; 62268-19-3 (S-isomer tartrate); 55511-44-9 (R-isomer); 57344-88-4 (R-isomer tartrate);
|
|
| PubChem CID |
2145
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
457.4±45.0 °C at 760 mmHg
|
|
| 熔点 |
152-154 °C(lit.)
|
|
| 闪点 |
230.4±28.7 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.566
|
|
| LogP |
1.41
|
|
| tPSA |
72.19
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
321
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CCC1(C2=CC=C(N)C=C2)CCC(NC1=O)=O
|
|
| InChi Key |
ROBVIMPUHSLWNV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
|
|
| 化学名 |
3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.76 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.76 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.76 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO +30% polyethylene glycol+1% Tween 80 : 8 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3051 mL | 21.5257 mL | 43.0515 mL | |
| 5 mM | 0.8610 mL | 4.3051 mL | 8.6103 mL | |
| 10 mM | 0.4305 mL | 2.1526 mL | 4.3051 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。