| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
PDE-4/phosphodiesterase (IC50 = 74 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
Apremilast (CC-10004) 的 IC50 为 104 nM (pIC50=6.98±0.2),可抑制脂多糖 (LPS) 释放 TNF-α。这与 Apremilast 对 PDE4 酶抑制的效力 (IC50=74 nM) 相似,并且几乎完全复制了 Apremilast 先前显示的对外周血单核细胞 (PBMC) 的抑制作用 (IC50=110 nM)。随着细胞内 cAMP 水平的增加,阿普斯特抑制 TNF-α,这些结果令人信服地支持了这一理论。在 PKA、Epac1 或 Epac2 敲低的情况下,未观察到阿普斯特诱导的 IL-10 激活和 TNF-α 抑制]。
|
||
| 体内研究 (In Vivo) |
当以 5 mg/kg 的剂量口服时,阿普斯特 (CC-10004) 使气囊中产生的 TNF-α 量显着减少 39%(载体的 61±6%,P <0.001),并降低白细胞数量减少 28%(载体的 72±12%,P <0.05)。免疫组织学研究证实,Apremilast 显着减少气囊膜中中性粒细胞的积累。甲氨蝶呤 (MTX) 和阿普斯特均可显着降低小鼠气囊模型中的白细胞浸润,但只有阿普斯特显着抑制 TNF-α 释放。当 MTX (1 mg/kg) 添加到 Apremilast (5 mg/kg) 中时,对白细胞浸润或 TNF-α 释放的抑制并不比单独使用 Apremilast 时更大[1]。已证明新型口服 PDE4 抑制剂阿普斯特可控制炎症介质。口服阿普司特后的平均最大血浆浓度(Cmax)测定为67.00±14.87 ng/mL。阿普司特的血浆浓度迅速下降,最终从血浆中消失,终末半衰期为0.92±0.46 h[2]。
|
||
| 酶活实验 |
Luminex分析[1]
使用Luminex x-MAP技术对细胞因子和趋化因子进行定量。使用Milliplex多分析物磁珠板分析组织培养上清液和小鼠渗出液中IL-1α、IL-6和IL-10的表达。根据试剂盒方案,使用适当的基质溶液(分别用于上清液和渗出物的培养基或PBS)进行检测。在Luminex 200仪器上收集数据,并使用Analyst 5.1软件进行四参数逻辑斯谛曲线拟合分析。对样品进行了两次化验。由制造商提供的已知参考细胞因子浓度生成的所有标准曲线的R2值计算为或接近1,回收率在80%至120%之间。每个试剂盒都按照预期进行了质量控制。 |
||
| 细胞实验 |
cAMP测量[1]
用直接cAMP ELISA试剂盒测定细胞内cAMP。将50%融合的Raw 264.7细胞饥饿24小时,以指定浓度的Apremilast (CC-10004)阿普司特 刺激30分钟,然后用脂多糖(LPS)刺激20分钟,并根据制造商的方案分析cAMP。 TNF-α测定[1] 在96孔板中生长264.7个原始细胞(100000个)。24小时后,用载体(终浓度为0.025%二甲亚砜(DMSO))或指定浓度的阿普司特(CC-10004)刺激细胞。30分钟后,用1μg/ml的LPS刺激细胞4小时。在研究CGS21680、SCH58261、ZM241385、BAY60-6583或GS6201时,在预末用药前15分钟加入腺苷受体配体。在安必利司特前24小时和1小时加入甲氨蝶呤。然后收集上清液,并按照制造商的说明用小鼠TNF-αQuantikine ELISA试剂盒定量TNF-α水平。 蛋白质印迹[1] 将70%融合的Raw 264.7细胞饥饿24小时,用Apremilast (CC-10004)刺激30分钟,然后用LPS刺激不同时间点(n=4),用放射免疫沉淀法(RIPA)缓冲液裂解细胞,用双辛可宁酸(BCA)测定蛋白质浓度。将蛋白质(4μg)进行7.5或10.0%SDS-PAGE,并转移到硝化纤维膜上。用TBS/Tween-20 0.05-3%BSA阻断非特异性结合。将膜与原代兔多克隆抗pCREB、小鼠单克隆抗CREB、兔多克隆反PDE4和小鼠单克隆抗肌动蛋白(各1:1000)孵育过夜(4°C)。在黑暗中,将膜与山羊抗兔IRDye 800CW 1:10000和山羊抗小鼠IRDye 680 RD 1:10000一起孵育。通过检测近红外荧光的Li cor Odyssey设备对蛋白质进行可视化。由于每种二次抗体都会发出不同光谱的信号,因此与一次抗体孵育同时进行肌动蛋白复制(以检查所有泳道都装载了相同量的蛋白质)。使用Image Studio 2.0.38软件通过密度分析对相应条带的强度进行定量。带强度的变化表示为未受刺激对照的百分比,以尽量减少不同实验之间的差异。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
An oral dose of apremilast is well-absorbed and the absolute bioavailability is approximately 73%. Tmax is approximately 2.5 hours and Cmax has been reported to be approximately 584 ng/mL in one pharmacokinetic study. Food intake does not appear to affect apremilast absorption. Only 3% and 7% of an apremilast dose are detected in the urine and feces as unchanged drug, respectively, indicating extensive metabolism and high absorption. The average apparent volume of distribution (Vd) is about 87 L, suggesting that apremilast is distributed in the extravascular compartment. In healthy patients, the plasma clearance of apremilast is about 10 L/hour. Human plasma protein binding of apremilast is approximately 68%. Mean apparent volume of distribution (Vd) is 87 L. The lacteal excretion of apremilast was evaluated following oral administration of apremilast to lactating CD-1 mice. In this study, female mice approximately 13 days postpartum received a single oral dose of apremilast at 10 mg/kg, administered by oral gavage in a volume of 10 mL/kg. Milk and blood samples from 5 animals per time point were obtained at 1, 6, and 24 hr postdose and apremilast concentrations determined in plasma and milk using LC-MS/MS analysis. The mean apremilast plasma concentrations at 1 and 6 hr post-dose were 984 and 138 ng/mL, while concentrations in milk were 1441 and 186 ng/mL, respectively. The resulting mean milk-to-plasma ratios ranged from 1.46 to 1.62, indicating transfer of apremilast into milk in mice. Plasma and milk concentrations were below the detection limit of 3 ng/mL in the 24-hr samples. In monkeys, pregnant animals were administered daily oral doses of apremilast beginning on gestation day 20 through gestation day 50, and a single oral dose on gestation day 100 at dosages of 20, 50, 200, and 1000 mg/kg/day (n = 16/group at the beginning of the study). Maternal and fetal blood was collected at 5 hr postdose on gestation Day 100. In all dosage groups, the fetal-to-maternal plasma concentration ratios were between 0.3 and 0.4, indicating apremilast crossed the placenta in monkeys. As part of fertility and developmental toxicity study in female CD-1 mice and an embryo-fetal development study in cynomolgus monkeys, the transport of apremilast across the placenta was assessed. In mice, following daily oral administration of apremilast beginning 15 days prior to cohabitation and continuing through Day 15 of presumed gestation at doses of 10, 20, 40, and 80 mg/kg/day, blood was collected from pregnant mice (n = 3/time point) at 0.5, 2, 4, 8, and 24 hr postdose on gestation Day 15. Blood was collected from fetuses) at the time of sacrifice in the 24 hr postdose mice. Maternal plasma apremilast concentrations increased in a less than dose proportional manner. The fetal plasma concentrations at 24 hr were highly variable, with six of the ten litters evaluated being below the limit of quantification (1 ng/mL). In fetal plasma from four of the ten litters evaluated, apremilast was quantified, with concentrations ranging from 14.5 to 2813 ng/mL. The mean fetal-to-maternal plasma concentration ratios ranged from 0.3 to 1.07, indicating apremilast crossed the placenta in mice. For more Absorption, Distribution and Excretion (Complete) data for Apremilast (13 total), please visit the HSDB record page. Metabolism / Metabolites Apremilast is heavily metabolized by various pathways, which include oxidation, hydrolysis, in addition to conjugation. About 23 metabolites are produced from its metabolism. The CYP3A4 primarily mediates the oxidative metabolism of this drug, with smaller contributions from CYP1A2 and CYP2A6 enzymes. The main metabolite of apremilast, M12, is an inactive glucuronide conjugate form of the O-demethylated drug. Some other major metabolites, M14 and M16, are significantly less active in the inhibition of PDE4 and inflammatory mediators than their parent drug, apremilast. After an oral dose, unchanged apremilast (45%) and the inactive metabolite, O-desmethyl apremilast glucuronide (39%) are found in the plasma. Minor metabolites M7 and M17 are active, but are only present in about 2% or less of apremilast concentrations, and likely not significant contributors to the actions of apremilast. The plasma clearance of apremilast is about 10 L/hr in healthy subjects, with a terminal elimination half-life of approximately 6-9 hours. Following oral administration of radio-labeled apremilast, about 58% and 39% of the radioactivity is recovered in urine and feces, respectively, with about 3% and 7% of the radioactive dose recovered as apremilast in urine and feces, respectively. Following oral administration in humans, apremilast is a major circulating component (45%) followed by inactive metabolite M12 (39%), a glucuronide conjugate of O-demethylated apremilast. It is extensively metabolized in humans with up to 23 metabolites identified in plasma, urine and feces. Apremilast is metabolized by both cytochrome (CYP) oxidative metabolism with subsequent glucuronidation and non-CYP mediated hydrolysis. In vitro, CYP metabolism of apremilast is primarily mediated by CYP3A4, with minor contributions from CYP1A2 and CYP2A6. In /an/ oral study, concentrations of both total radioactivity (e.g., parent compound plus any metabolites) and of parent compound in plasma were greater in females than in males. In males, the total radioactivity AUC values were 25 to 96 times greater than those for parent compound, whereas in females the difference was only 2- to 3-fold, suggesting that metabolism was more extensive in male than in female rats. In the same study following six daily doses, accumulation was indicated by Cmax and AUC values in females, but not in males. In a bile-duct cannulated male mouse study following a single 10 mg/kg oral dose of (14)C-apremilast, 54% and 16% of the radioactive dose was excreted via the biliary and urinary routes, suggesting that at least 70% of the radioactive dose was absorbed in mice, indicating apremilast is subject to moderate first pass metabolism. Toxicokinetic evaluation in mice suggests exposure increases dose-proportionally and less than dose-proportionally at doses over 100 mg/kg/day. The studies do not indicate sex-related differences or inversion of apremilast to its R enantiomer in mice. For more Metabolism/Metabolites (Complete) data for Apremilast (6 total), please visit the HSDB record page. Biological Half-Life The average elimination half-life of this drug ranges from 6-9 hours. terminal elimination half-life of approximately 6-9 hours |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Apremilast is a white to pale-yellow powder. Apremilast is used for the treatment of adult patients with active psoriatic arthritis. It is also used for the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. HUMAN EXPOSURE AND TOXICITY: The most common adverse reactions are diarrhea, nausea, upper respiratory tract infection, and headache, including tension headache. Apremilast did not induce chromosome aberrations in cultured human peripheral blood lymphocytes in the presence or absence of metabolic activation. ANIMAL STUDIES: Apremilast has low potential for acute toxicity. Apremilast was evaluated in a series of repeat-dose oral toxicity studies of up to 6 months duration in mice (dose levels of 10, 100 and 1000 mg/kg/day), 12 months duration in monkeys (dose levels of 60, 180 and 600 mg/kg/day) and 90 days duration in rats. Apremilast-related mortality was observed in mice and rats and was primarily attributed to vascular and/or perivascular inflammation. Dose-related inflammatory responses were predominantly observed in mice and rats and included neutrophilia, lymphopenia, and changes in serum proteins (decreased albumin, increased globulin, and increased hapotoglobin, C-reactive protein (CRP), and/or fibrinogen). These inflammatory responses were associated with arteritis and perivascular inflammation in various tissues and organs (e.g. mesentery, heart, lungs, thymus, liver, skeletal muscle, mammary gland, skin and pancreas) in mice and rats, but not in monkeys, even at higher systemic exposures than those achieved in mice and rats. Complete or partial reversibility of the inflammatory findings in mice and rats was observed. Other target organs of apremilast toxicity include non-adverse centrilobular hepatocellular hypertrophy in the liver (mouse) and variable lymphoid depletion in lymphoid tissues (mouse and rat). Long-term studies were conducted in mice and rats with apremilast to evaluate its carcinogenic potential. No evidence of apremilast-induced tumors was observed in mice at oral doses up to 8.8-times the Maximum Recommended Human Dose (MRHD) on an AUC basis (1000 mg/kg/day) or in rats at oral doses up to approximately 0.08- and 1.1-times the MRHD, (20 mg/kg/day in males and 3 mg/kg/day in females, respectively). In a male mouse fertility study, apremilast at oral dosages of 1, 10, 25, and 50 mg/kg/day produced no effects on male fertility. In a combined female mouse fertility and embryo-fetal developmental toxicity study with oral dosages of 10, 20, 40, and 80 mg/kg/day, altered estrous cycling and increased time to mating was observed from 20 mg/kg/day. Nevertheless, all mice mated and pregnancy rates were unaffected. Apremilast did not induce mutations in an Ames assay. Apremilast was not clastogenic in an in vivo mouse micronucleus assay at doses up to 2000 mg/kg/day. Interactions Otezla has not been evaluated and is not indicated to be used in combination with biological therapeutics for psoriasis such as TNF antagonists and anti-IL-12/23 p40 antibodies. Otezla is not recommended in combination with these biological therapeutics. Otezla has not been evaluated and is not indicated in combination with potent immunosuppressive drugs (e.g. cyclosporine, tacrolimus). Otezla is not recommended in combination with potent immunosuppressive drugs. Apremilast exposure (AUC) and maximal concentrations (Cmax) were decreased by 72% and 43% when co-administered with CYP3A4 inducer rifampin, and may result in reduced clinical efficacy of apremilast. Hence coadministration of rifampin or other CYP3A4 inducers (e.g. phenobarbital, carbamazepine, phenytoin) along with Otezla is not recommended. St John's Wort is a CYP3A4 inducer, and co-administration with Otezla may result in loss of efficacy or reduced clinical response, and is not recommended. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Anti-Inflammatory Agents, Non-Steroidal; Phosphodiesterase Inhibitors Otezla is indicated for the treatment of adult patients with active psoriatic arthritis. /Included in US product label/ Otezla is indicated for the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. /Included in US product label/ EXPL THER /The purpose of this study is/ to evaluate the efficacy and safety of an oral phosphodiesterase 4 inhibitor, apremilast, in treatment of ankylosing spondylitis (AS) by monitoring symptoms and signs in a pilot study including exploratory investigation of effects of PDE4 inhibition on blood biomarkers of bone biology. In this double-blind, placebo-controlled, single-centre, Phase II study, patients with symptomatic AS with active disease on MRI were randomized to apremilast 30 mg BID or placebo over 12 weeks. Bath Indices were monitored serially. Patients were followed for 4 weeks after stopping medication. Bone biomarkers were assessed at baseline and day 85. 38 subjects were randomised and 36 subjects completed the study. Although the primary end-point (change in BASDAI at week 12) was not met, apremilast was associated with numerically greater improvement from baseline for all clinical assessments compared with placebo with mean change in BASDAI (-1.59 + or - 1.48 vs -0.77 + or - 1.47), BASFI (-1.74 + or - 1.91 vs -0.28 + or - 1.61) and BASMI (-0.51 + or - 1.02 vs -0.21 + or - 0.67); however, differences did not achieve statistical significance. The clinical indices returned to baseline values by 4 weeks after cessation of apremilast. Six apremilast patients (35.3%) vs 3 placebo (15.8%) achieved ASAS20 responses (p=0.25). There were statistically significant decreases in serum RANKL and RANKL:osteoprotegrin ratio and plasma sclerostin but no significant changes in serum DKK-1, bone alkaline phosphatase, TRAP5b, MMP3, osteoprotegrin, or osteocalcin. Although a small pilot study, these results suggest that apremilast may be effective and well tolerated in AS and modulates biomarkers of bone biology. These data support further research of apremilast in axial inflammation. EXPL THER Discoid lupus erythematosus (DLE) is a chronic inflammatory disorder mediated by Th1 cells. Apremilast is a novel oral PDE4 enzyme inhibitor capable of blocking leukocyte production of IL-12, IL-23, TNF-a, INF- with subsequent suppression of Th1 and Th17-mediated immune responses, and proven clinical efficacy for psoriasis as well as rheumatoid and psoriatic arthritis. Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) showed a significant (P<0.05) decrease after 85 days of treatment with apremilast 20 mg twice daily in 8 patients with active discoid lupus. The adverse events related to the drug were mild and transient. This is the first open label study to use apremilast as a treatment modality for discoid lupus. Our observations indicate that apremilast may constitute a safe and effective therapeutic option for DLE. Drug Warnings Treatment with Otezla is associated with an increase in adverse reactions of depression. Before using Otezla in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment with Otezla in such patients. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider. Prescribers should carefully evaluate the risks and benefits of continuing treatment with Otezla if such events occur. The safety and effectiveness of Otezla in pediatric patients less than 18 years of age have not been established. It is not known whether Otezla or its metabolites are present in human milk; however apremilast was detected in milk of lactating mice. Because many drugs are present in human milk, caution should be exercised when Otezla is administered to a nursing woman. FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./ For more Drug Warnings (Complete) data for Apremilast (11 total), please visit the HSDB record page. Pharmacodynamics Apremilast reduces but does not completely inhibit various inflammatory cytokines such as IL-1α, IL-6, IL-8, IL-10 MCP-1, MIP-1β, MMP-3, and TNF-α, relieving the symptoms of psoriasis and Behcet's disease, which are caused by an increase in these inflammatory mediators. This drug has also been proven to be effective in relieving the pain associated with oral ulcers in Behcet's disease. Apremilast may cause unwanted weight loss and worsen depression, leading to suicidal thoughts or actions. It is advisable to monitor for symptoms of depression and seek medical attention if they occur, especially in patients with pre-existing depression. The need for apremilast should be carefully assessed along with the risk of worsening depression and suicide. If weight loss occurs, the degree of weight loss should be evaluated, and consideration should be made for the possible discontinuation of apremilast. Introduction: This work was undertaken to delineate intracellular signaling pathways for the PDE4 inhibitor apremilast and to examine interactions between apremilast, methotrexate and adenosine A2A receptors (A2AR). Methods: After apremilast and LPS incubation, intracellular cAMP, TNF-α, IL-10, IL-6 and IL-1α were measured in the Raw264.7 monocytic murine cell line. PKA, Epac1/2 (signaling intermediates for cAMP) and A2AR knockdowns were performed by shRNA transfection and interactions with A2AR and A2BR, as well as with methotrexate were tested in vitro and in the murine air pouch model. Statistical differences were determined using one or two-way ANOVA or Student's t test. The alpha nominal level was set at 0.05 in all cases. A P value of < 0.05 was considered significant. Results: In vitro, apremilast increased intracellular cAMP and inhibited TNF-α release (IC50=104nM) and the specific A2AR-agonist CGS21680 (1μM) increased apremilast potency (IC50=25nM). In this cell line, apremilast increased IL-10 production. PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by apremilast. In the murine air pouch model, both apremilast and MTX significantly inhibited leukocyte infiltration, while apremilast, but not MTX, significantly inhibited TNF-α release. The addition of MTX (1 mg/kg) to apremilast (5 mg/kg) yielded no more inhibition of leukocyte infiltration or TNF-α release than with apremilast alone. Conclusions: The immunoregulatory effects of apremilast appear to be mediated by cAMP through the downstream effectors PKA, Epac1, and Epac2. A2AR agonism potentiated TNF-α inhibition by apremilast, consistent with the cAMP-elevating effects of that receptor. Because the A2AR is also involved in the anti-inflammatory effects of MTX, the mechanism of action of both drugs involves cAMP-dependent pathways and is therefore partially overlapping in nature.[1] A rapid, sensitive and selective ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS-MS) was developed and validated for the determination and pharmacokinetic investigation of apremilast in rat plasma. Sample preparation was accomplished through a simple one-step deproteinization procedure with 0.2 mL of acetonitrile to a 0.1 mL plasma sample. Plasma samples were separated by UPLC on an Acquity UPLC BEH C18 column using a mobile phase consisting of acetonitrile-0.1% formic acid in water with gradient elution. The total run time was 3.0 min, and the elution of apremilast was at 1.27 min. The detection was performed on a triple quadrupole tandem mass spectrometer in the multiple reaction-monitoring mode using the respective transitions m/z 461.3 → 257.1 for apremilast and m/z 237.2 → 194.2 for carbamazepine (internal standard). The calibration curve was linear over the range of 0.1-100 ng/mL with a lower limit of quantitation of 0.1 ng/mL. The mean recovery of apremilast in plasma was in the range of 83.2-87.5%. Both intraday and interday precision were <9.6%. This method was successfully applied in the pharmacokinetic study after oral administration of 6.0 mg/kg apremilast in rats.[2] |
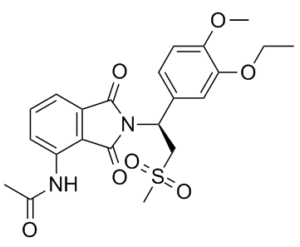
| 分子式 |
C22H24N2O7S
|
|---|---|
| 分子量 |
460.50
|
| 精确质量 |
460.13
|
| 元素分析 |
C, 57.38; H, 5.25; N, 6.08; O, 24.32; S, 6.96
|
| CAS号 |
608141-41-9
|
| 相关CAS号 |
Apremilast-d5;1258597-47-5; (R)-Apremilast; 608141-44-2;(Rac)-Apremilast-d5; 1258597-61-3; 253168-86-4
|
| PubChem CID |
11561674
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
741.3±60.0 °C at 760 mmHg
|
| 闪点 |
402.1±32.9 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.612
|
| LogP |
1.75
|
| tPSA |
130.95
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
825
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CCOC1=C(C=CC(=C1)[C@@H](CS(=O)(=O)C)N2C(=O)C3=C(C2=O)C(=CC=C3)NC(=O)C)OC
|
| InChi Key |
IMOZEMNVLZVGJZ-QGZVFWFLSA-N
|
| InChi Code |
InChI=1S/C22H24N2O7S/c1-5-31-19-11-14(9-10-18(19)30-3)17(12-32(4,28)29)24-21(26)15-7-6-8-16(23-13(2)25)20(15)22(24)27/h6-11,17H,5,12H2,1-4H3,(H,23,25)/t17-/m1/s1
|
| 化学名 |
(S)-N-(2-(1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide
|
| 别名 |
CC-10004; 608141-41-9; OTEZLA; CC-10004; CC 10004; Apremilast (CC-10004); CC10004; CHEBI:78540; CC10004; Apremilast; CC 10004; Otezla (Trade name)
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (5.43 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5 mg/mL (10.86 mM) in 0.5% CMC-Na 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1716 mL | 10.8578 mL | 21.7155 mL | |
| 5 mM | 0.4343 mL | 2.1716 mL | 4.3431 mL | |
| 10 mM | 0.2172 mL | 1.0858 mL | 2.1716 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06324435 | Not yet recruiting NEW | Drug: Apremilast | Alcohol Use Disorder | Yale University | April 15, 2024 | Phase 1 |
| NCT03656666 | Active, not recruiting | Drug: Apremilast Drug: Placebo | Lichen Planus of Vulva Female Genital Disease |
Oslo University Hospita | September 24, 2019 | Phase 2 |
| NCT04804553 | Recruiting | Drug: Apremilast Drug: Placebo | Active Juvenile Psoriatic Arthritis | Amgen | March 17, 2022 | Phase 3 |
| NCT04528082 | Recruiting | Drug: Apremilast Drug: Placebo | Behçet Disease | Amgen | September 9, 2021 | Phase 3 |
Apremilast and methotrexate (MTX) prevent inflammation in the air pouch independently.Arthritis Res Ther.2015 Sep 15;17:249. |
|---|
Apremilast inhibits lipopolysaccharide (LPS)-induced TNF-α release via cyclic adenosine monophosphalphate (cAMP).Arthritis Res Ther.2015 Sep 15;17:249. |
Effect of Protein kinase A (PKA), Exchange protein directly activated by cAMP (Epac)1 and Epac2 knockdown on the action of apremilast.Arthritis Res Ther.2015 Sep 15;17:249. |