| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
cIAP1 (Ki = 1.9 nM); cIAP2 (Ki = 5.1 nM); XIAP (Ki = 66.4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
AT-406 是一种 Smac 模拟物,在与 XIAP 的氢键作用和疏水相互作用方面似乎都非常模拟 AVPI 肽,并且与 XIAP 的 W323 具有额外的疏水接触。与 Smac AVPI 肽相比,AT-406(1 μM)的结合亲和力高出 50-100 倍。当 caspase-9 在无细胞环境中被 500 nM XIAP BIR3 抑制时,AT-406 会完全逆转这种效应。 AT-406 会降低细胞 XIAP 蛋白,同时还会导致 cIAP1 蛋白在 MDA-MB-231 细胞中快速降解。在 MDA-MB-231 细胞和 SK-OV-3 卵巢细胞中的 IC50 值分别为 144 和 142 nM,对 MCF-12F 细胞(类似于正常人乳腺上皮细胞)和原代人正常前列腺上皮细胞具有低毒性,AT-406有效抑制多种人类癌细胞系。 AT-406 通过激活 caspase-3 和裂解 PARP,引起 MDA-MB-231 细胞凋亡。 [1]
|
| 体内研究 (In Vivo) |
在小鼠、大鼠、非人灵长类动物和狗中,AT-406 具有良好的口服生物利用度和药代动力学 (PK) 特性。 AT-406 在 100 mg/kg 剂量下可有效诱导肿瘤组织中的 cIAP1 降解、procaspase-8 加工和 PARP 裂解,即使在 200 mg/kg 剂量下,AT-406 在 MDA-MB-231 异种移植物中也具有良好的耐受性。 100 mg/kg 时,AT-406 显着抑制肿瘤生长,p 值为 0.0012。 [1]
|
| 酶活实验 |
FL-AT-406(荧光标记的 AT-406)用于开发一套新的 FP 测定法,用于测定 Smac 模拟物与 XIAP、cIAP-1 和 cIAP-2 BIR3 蛋白的结合亲和力。使用固定浓度的 FL-AT-406 和不同浓度的蛋白质直至完全饱和的滴定实验用于计算 FL-AT-406 对每种 IAP 蛋白质的 Kd 值。 Microflu 2 96 孔黑色圆底板用于使用 Infinite M-1000 读板器测量荧光偏振值。对于 XIAP BIR3、cIAP-1 BIR3 和 cIAP-2 BIR3 的实验,将 FL-AT-406(每孔分别为 2、1 和 1 nM)和各种蛋白质浓度添加到终体积为 125 μL 的溶液中。测定缓冲液(100 mM 磷酸钾,pH 7.5,100 g/mL 牛球蛋白,0.02% 叠氮化钠,含 4% DMSO)。彻底混合后,将板在室温下轻轻摇动两到三个小时。在 485 nm 的激发波长和 530 nm 的发射波长下,测量以毫偏振单位 (mP) 为单位的偏振值。然后,使用 Graphpad Prism 5.0 软件,通过拟合 S 形剂量依赖性 FP 增加作为蛋白质浓度的函数来计算平衡解离常数 (Kd)。在 XIAP3 BIR3 的竞争性结合测试中,AT-406 与 20 nM XIAP BIR3 蛋白和 2 nM FL-AT-406 在测定缓冲液(100 mM 磷酸钾,pH 7.5;100 μg/mL 牛 γ-球蛋白;0.02 %叠氮化钠)。实验中使用 3 nM 蛋白质和 1 nM FL-AT-406 来确定 cIAP1 BIR3 蛋白质的竞争性结合。 5 nM 蛋白质和 1 nM FL-AT-406 用于 cIAP2 BIR3 的竞争性结合测试。使用 Infinite M-1000 读板器,在孵育两到三个小时后确定每个竞争性结合实验的偏振值。使用非线性最小二乘分析,从图中提取 IC50 值或 50% 结合示踪剂被取代时的抑制剂浓度。 PRISM 程序用于拟合曲线。
|
| 细胞实验 |
以 (3-4) × 103 个细胞/孔的密度,将细胞接种到含有 AT-406 的 96 孔平底细胞培养板中,并孵育 4 天。将三至四个 103 个细胞/孔的 AT-406 接种细胞置于 96 孔平底细胞培养板中,然后将细胞孵育四天。通过(2-(2-甲氧基-4-硝基苯基)-3-(4-硝基苯基)-5-(2,4-二磺基苯基)测定来测定不同浓度AT-406处理后的细胞生长抑制率-2H-四唑单钠盐(WST-8)。将WST-8添加到每个孔中至终浓度10%,然后将板在37°C下孵育2−3小时。使用TECAN ULTRA读数器,计算样品在450 nm处的吸光度,通过比较未经处理的细胞和经AT-406处理的细胞的吸光度,可以确定抑制细胞生长50%(IC50)的AT-406浓度。使用TECAN ULTRA读数仪测量样品在450 nm处的吸光度,通过比较处理和未处理细胞的吸光度,可以确定AT-406抑制50%细胞生长的浓度(IC50)。
|
| 动物实验 |
MDA-MB-231 xenograft tumors in severe combined immune deficiency (SCID) mice
10 mg/kg (i.v.), 10 mg/kg (p.o.), 30 mg/kg (p.o.) and 100 mg/kg (p.o.) Administered via intravenously (i.v.) or oral gavage (p.o.) In Vivo pharmacodynamic (PD) studies[1] For in vivo PD studies, the MDA-MB-231 xenograft tumor model was employed. To develop xenograft tumors, 5 × 106 MDA-MB-231 cancer cells with matrigel were injected subcutaneously on the dorsal side of the severe combined immunodeficient mice (SCID mice from Charles River), one tumor per mouse. Mice bearing MDA-MB-231 xenograft tumors were administered with a single dose of AT406 (Xevinapant, SM406, ARRY334543) in its HCl salt form at 100 mg/kg via oral gavage, Taxotere at 7.5 mg/kg intravenously or vehicle control. Tumor tissues were harvested at indicated time points. Tumor tissues were analyzed using Western blotting to examine levels of cIAP1 and XIAP, caspase-8 processing and PARP cleavage in tumor tissues. In Vivo Pharmacokinetic studies in plasma and MDA-MB-231 tumor tissues in SCID mice[1] To develop xenograft tumors, 5 × 106 MDA-MB-231 cancer cells with matrigel were injected subcutaneously on the dorsal side of the severe combined immunodeficient mice (SCID mice from Charles River), two tumors (left and right sides) per mouse. Mice bearing MDA-MB-231 xenograft tumors were administered with a single dose of compound 2 [AT406 (Xevinapant, SM406, ARRY334543)] in its HCl salt form at 100 mg/kg via oral gavage. Blood and tumor samples were collected from each mouse by terminal cardiac puncture at 0.25, 0.5, 1, 2, 4, 6, 8, 24 h post-dose. Samples were taken from three mice at each time point. Blood samples were collected into potassium heparin treated tubes and centrifuged at 2000g and 4°C for 10 min. Plasma was collected and stored at −80°C prior to analysis. Isolated tumor tissues were immediately frozen and ground with a mortar and pestle in liquid nitrogen, then stored at −80°C until analysis. In Vivo antitumor efficacy study[1] SCID mice (8–10 per group) bearing MDA-MB-231 xenograft tumors were treated with different doses of AT406 (Xevinapant, SM406, ARRY334543), or 7.5 mg/kg of Taxotere or vehicle control daily, 5 days a week for 2 weeks. Tumor sizes and animal weights were measured 3 times a week during the treatment and twice a week after the treatment. Data are presented as mean tumor volumes ± SEM. Statistical analyses were performed by two-way ANOVA and unpaired two-tailed t test, using Prism (version 4.0, GraphPad, La Jolla, CA). P < 0.05 was considered statistically significant. The efficacy experiment was performed under the guidelines of the University of Michigan Committee for Use and Care of Animals. Pharmacokinetics of AT406 (Xevinapant, SM406, ARRY334543) in rats, dogs and non-human primates[1] Pharmacokinetic (PK) studies in male Sprague Dawley rats, beagle dogs and cynomolgus monkeys (non-human primates) were performed a CRO company. AT406 (Xevinapant, SM406, ARRY334543) in its hydrochloride salt form was used in pharmacokinetic (PK) evaluations and was dissolved in saline to yield final concentration at 25 mg/mL (pH≈7). The solution was administered to animals on preparation. The concentration of AT406 (Xevinapant, SM406, ARRY334543) in dosing solution was confirmed by HPLC. For PK studies in rats, dogs and monkeys, animals were randomly assigned to the treatment groups and were carotid cannulated before the PK studies. The LC system comprised an Agilent liquid chromatograph equipped with an isocratic pump (1100 series), an autosampler (1100 series) and a degasser (1100 series). Mass spectrometric analysis was performed using an API3000 (triple-quadruple) instrument from AB Inc with an ESI interface. The data acquisition and control system were created using Analyst 1.4 software from ABI Inc. The concentrations in plasma below the limit of quantitation (LOQ = 5 ng/mL) were designated as zero. The pharmacokinetic data analysis was performed using noncompartmental analysis. Oral bioavailability was calculated as F(%)=(Dose(oral)×AUC(0-∞)(oral))/(Dose (iv)×AUC(0-∞) (iv))*100%. |
| 参考文献 | |
| 其他信息 |
Xevinapant is an orally available mimetic of the natural second mitochondrial-derived activator of caspases (Smac) and inhibitor of Inhibitor of Apoptosis Proteins (IAPs), with potential immunomodulating, apoptotic-inducing, chemo-radio-sensitizing and antineoplastic activities. Upon oral administration,xevinapant targets and binds to the Smac binding groove on IAPs, including the direct caspase inhibitor X chromosome-linked IAP (XIAP), and the cellular IAPs 1 (c-IAP1) and 2 (c-IAP2). This inhibits the activities of these IAPs and promotes the induction of apoptosis. Additionally, as xevinapant inhibits the activity of IAPs, it may work synergistically with cytotoxic drugs and/or radiation to overcome tumor cell resistance to apoptosis. As IAPs regulate nuclear factor-kappa B (NFkB) signaling pathways, which drives the expression of genes involved in immune and inflammatory responses, xevinapant may enhance anti-tumor immune responses when administered with certain immunomodulating agents, such as immune checkpoint inhibitors. IAPs are overexpressed by many cancer cell types and suppress both intrinsic and extrinsic apoptosis by binding to and inhibiting active caspases via their baculoviral lAP repeat (BIR) domains. They contribute to chemo-radio-resistance of cancer cells to certain cytotoxic agents and radiation, promote tumor cell survival and are associated with poor prognosis in certain types of cancer. SMAC, a pro-apoptotic mitochondrial protein, is an endogenous inhibitor of the IAPs family of cellular proteins.
See also: Xevinapant Hydrochloride (is active moiety of). Drug Indication Treatment of head and neck epithelial malignant neoplasms |
| 分子式 |
C32H43N5O4
|
|---|---|
| 分子量 |
561.71
|
| 精确质量 |
561.331
|
| 元素分析 |
C, 68.42; H, 7.72; N, 12.47; O, 11.39
|
| CAS号 |
1071992-99-8
|
| 相关CAS号 |
Xevinapant hydrochloride;1071992-57-8
|
| PubChem CID |
25022340
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
840.5±65.0 °C at 760 mmHg
|
| 闪点 |
462.1±34.3 °C
|
| 蒸汽压 |
0.0±3.1 mmHg at 25°C
|
| 折射率 |
1.603
|
| LogP |
2.09
|
| tPSA |
117.83
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
896
|
| 定义原子立体中心数目 |
4
|
| SMILES |
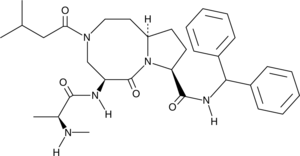
O=C1[C@]([H])(C([H])([H])N(C(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])([H])C([H])([H])[C@@]2([H])C([H])([H])C([H])([H])[C@@]([H])(C(N([H])C([H])(C3C([H])=C([H])C([H])=C([H])C=3[H])C3C([H])=C([H])C([H])=C([H])C=3[H])=O)N21)N([H])C([C@]([H])(C([H])([H])[H])N([H])C([H])([H])[H])=O
|
| InChi Key |
LSXUTRRVVSPWDZ-MKKUMYSQSA-N
|
| InChi Code |
InChI=1S/C32H43N5O4/c1-21(2)19-28(38)36-18-17-25-15-16-27(37(25)32(41)26(20-36)34-30(39)22(3)33-4)31(40)35-29(23-11-7-5-8-12-23)24-13-9-6-10-14-24/h5-14,21-22,25-27,29,33H,15-20H2,1-4H3,(H,34,39)(H,35,40)/t22-,25+,26-,27-/m0/s1
|
| 化学名 |
(5S,8S,10aR)-N-benzhydryl-5-[[(2S)-2-(methylamino)propanoyl]amino]-3-(3-methylbutanoyl)-6-oxo-1,2,4,5,8,9,10,10a-octahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide
|
| 别名 |
DeBio-1143; DeBio1143; DeBio 1143; Xevinapant; AT 406; AT-406; AT406; SM406; SM 406; N65WC8PXDD; SM-406; UNII-N65WC8PXDD; Xevinapant; ARRY-334543; ARRY 334543; ARRY334543
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7803 mL | 8.9014 mL | 17.8028 mL | |
| 5 mM | 0.3561 mL | 1.7803 mL | 3.5606 mL | |
| 10 mM | 0.1780 mL | 0.8901 mL | 1.7803 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01078649 | Completed | Drug: Debio 1143 (AT-406) |
Cancer Malignancy Lymphoma |
Debiopharm International SA | March 29, 2010 | Phase 1 |
|
|---|