| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|

| 靶点 |
Rac1
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:硫唑嘌呤抑制 Rac1 靶基因的激活,例如丝裂原激活蛋白激酶激酶 (MEK)、NF-kappaB 和 bcl-x(L),导致原代人 CD4+ T 淋巴细胞凋亡的线粒体途径。因此,硫唑嘌呤通过调节 Rac1 活性将共刺激信号转化为细胞凋亡信号。因此,硫唑嘌呤生成的 6-Thio-GTP 通过阻断 Rac 蛋白上的 Vav 活性来阻止有效免疫反应的发展。硫唑嘌呤 (1 mM) 可恢复 ATP 水平并阻止细胞损伤,而富含葡萄糖的培养基中的培养可增加 ATP 水平并改善细胞死亡。硫唑嘌呤在第 1 天降低活力 5-34%,在第 4 天降低 42-92%。硫唑嘌呤降低肝细胞的活力,并在分离的大鼠肝细胞原代培养物中诱导以下事件:细胞内减少谷胱甘肽 (GSH) 消耗、代谢活性降低、和乳酸脱氢酶的释放。硫唑嘌呤对肝细胞的作用与完整的离体大鼠肝线粒体的肿胀和耗氧量增加有关。
|
| 体内研究 (In Vivo) |
在小鼠-大鼠脑异种移植物中,硫唑嘌呤联合环孢菌素 A 和泼尼松龙可使 15 个移植物中的 14 个存活(93%),而单独使用环孢菌素 A 治疗组中的 14 个移植物中只有 11 个存活(79%)。
|
| 细胞实验 |
细胞系:大鼠肝细胞、人肝细胞
浓度:0-50 μM 孵育时间:24-48 小时 结果:低浓度0.5时,大鼠肝细胞的细胞活力和细胞内GSH水平下降μM,但在浓度低于 50 μM 时细胞活力没有显着降低,并且在人肝细胞中浓度低至 1 μM 时明显注意到 GSH 消耗。 |
| 动物实验 |
Outbred female CD-1 mice, Female ICR mice
25-400 mg/kg Oral gavage; everyday; 10days |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral azathioprine is well absorbed, with a Tmax of 1-2h. Further data regarding the absorption of azathioprine is not readily available. Azathioprine and mercaptopurine are not detectable in urine after 8 hours. Further data regarding the route of elimination of azathioprine are not available. Data regarding the volume of distribution of azathioprine is not readily available. Data regarding the clearance of azathioprine is not readily available. Azathioprine and mercaptopurine are moderately bound to plasma proteins and are partially dialyzable. They are rapidly removed from the blood by oxidation or methylation in the liver and/or erythrocytes. Renal clearance is of little impact in biological effectiveness or toxicity, but dose reduction is practiced in patients with renal failure. Azathioprine is well absorbed orally and reaches maximum blood levels within 1 to 2 hours after administration. Azathioprine is well absorbed from the gastrointestinal tract and has an oral bioavailibility of approximately 60%. Azathioprine is rapidly cleared from the blood; both azathioprine and mercaptopurine are approximately 30% bound to serum proteins, both appear dialyzable, and both appear to cross the placenta. The metabolites are excreted in the urine, largely as 6-mercaptopurine. Less than 2% of azathioprine and 20 to 40% of 6-mercaptopurine are excreted as unchanged drugs in the urine. Metabolism / Metabolites Azathioprine is converted to 6-mercaptopurine nonenzymatically. 6-mercaptopurine is then metabolized to 6-methylmercaptopurine by thiopurine methyltransferase, 6-thiouric acid by xanthine oxidase, or 6-thiosine-5'-monophosphate by hypoxanthine phosphoribosyltransferase. 6-thiosine-5'-monophosphate is metabolized to 6-methylthiosine-5'-monophosphate by thiopurine methyltransferase or 6-thioxanthylic acid by inosine monophosphate dehydrogenase. 6-thioxanthylic acid is metabolized by guanosine monophosphate synthetase to 6-thioguanine monophosphate, the first of the 6-thioguanine nucleotides. 6-thioguanine monophosphate is phosphorylated to produce the remaining 6-thioguanine nucleotides, 6-thioguanine diphosphate and 6-thioguanine triphosphate. Orally administered azathioprine is rapidly divided in vivo to form 6-mercaptopurine. Metabolized in vivo to 6-mercaptopurine, q.v. Azathioprine is metabolized to 6-mercaptopurine. Primarily converted to the active metabolites 6-mercaptopurine and 6-thioinosinic acid via a non-enzymatica process and glutathione transferases. Activation of 6-mercaptopurine occurs via hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and a series of multi-enzymatic processes involving kinases to form 6-thioguanine nucleotides (6-TGNs) as major metabolites Route of Elimination: Both compounds are rapidly eliminated from blood and are oxidized or methylated in erythrocytes and liver; no azathioprine or mercaptopurine is detectable in urine after 8 hours. Biological Half-Life The half life of azathioprine is approximately 5 hours. The elimination half-life of azathioprine is approximately 12 to 15 minutes, and that of 6-mercaptopurine is approximately 30 minutes to 4 hours. The total boby clearance of azathioprine is 60 ml/min/kg, and that of 6-mercaptopurine, 10 ml/min/kg. The half-life of azathioprine itself is about 10 minutes, and that for mercaptopurine is about an hour. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Group: Other Immunosuppressive agents. Azathioprine: pale yellow, odorless powder. The drug is insoluble in water and very slightly soluble in ethanol. HUMAN EXPOSURE: Summary: Main risks and target organs: Azathioprine is a myelotoxic and hepatotoxic immunosuppressive agent. Bone marrow and liver are the main targets but gastrointestinal tract, kidney, lungs, CNS and skin may also be affected. Transient gastroenteritis may be observed with massive overdose. Leukopenia is the main toxic effect which may occur during azathioprine therapy and in the overdose patient. Liver and kidney function tests may be altered but usually returned to normal after discontinuation of the drug. Summary of clinical effects: Oral ulceration occurs rarely with therapeutic doses but may be seen with large doses. Gastrointestinal disturbances such as nausea, vomiting, abdominal pain and diarrhea can appear mainly at higher doses. Acute pancreatitis was also reported following long term azathioprine treatment. Suppression of the bone marrow mainly leukopenia and occasionally pancytopenia may be seen after therapeutic doses and overdoses of azathioprine. Septic shock due to this immunosuppression may occur. Hepatic dysfunction (hepatocellular and cholestatic), venocclusive disease and hemangioma of the liver following azathioprine therapy were documented. Acute restrictive lung disease, interstitial nephritis and a case of progressive leukoencephalopathy after 4 years azathioprine therapy were reported. Skin rash, alopecia and urticaria and a case of palmar-plantar erythema with desquamation and pain were also documented. Diagnosis: Diagnosis of azathioprine overdose is based on history of the drug taken and clinical findings mainly gastrointestinal dysfunction, leukopenia and liver dysfunction. Peripheral cell blood counts and liver function tests are required. Estimation of 6-thioguanine nucleotide, a cytotoxic metabolite of azathioprine in red blood cell may confirm the diagnosis and could also be used to predict bone marrow toxicity of azathioprine. Indications: Uses: Azathioprine is used as an adjunct for the prevention of the rejection of kidney allografts. The drug is used in conjunction with other immunosuppressive therapy including local radiation therapy, corticosteroids, and other cytotoxic agents. Azathioprine may be used for the treatment of conditions which involve derangement of the immune system including chronic active hepatitis, severe rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis, pemphigus vulgaris, polyarteritis nodosa, acquired hemolytic anemia, Crohn's disease and idiopathic thrombocytopenia. Contraindications: Azathioprine is contraindicated in patients who are hypersensitive to the drug. Azathioprine is also contraindicated in those patients with renal failure, impaired hepatic function and in pregnant women. Routes of entry: Oral: Azathioprine is usually administered orally. Parenteral: Following renal transplantation, azathioprine may initially be given intravenously to patients unable to tolerate oral medication. Oral therapy should replace parenteral therapy as soon as possible. Kinetics: Absorption by route of exposure: Azathioprine is readily absorbed from the gastrointestinal tract with only 12.6% of the dose being detected in the stool over a 48 hour period. Distribution by route of exposure: Azathioprine is rapidly distributed throughout the body with peak plasma concentrations being reached at 1 to 2 hours after dosing. Small amounts of azathioprine bind to plasma proteins (to a maximum of 30%) and only very small amounts enter the brain. Azathioprine crosses the placenta and trace amounts of the 6-mercaptopurine metabolite have been detected in fetal blood. Biological half-life by route of exposure: The plasma half-life of azathioprine is 3 to 5 hours. Metabolism: Azathioprine is metabolized in vivo to mercaptopurine, apparently by sulfhydryl compounds such as glutathione. Mercaptopurine is oxidized and methylated to several derivatives among which 6-thiouric acid predominates; the proportion of metabolites varies amongst individuals. The fate of the nitromethylimidazole portion of azathioprine has not been completely elucidated. Small amounts of azathioprine are also split to give 1-methyl-4-nitro-5-thioimidazole. The active metabolites, 6-thioguanine nucleotides, responsible for the therapeutic action, are formed intracellularly and appear to have very long half-lives. Elimination by route of exposure: The metabolites of azathioprine are excreted by the kidneys; only small amounts of azathioprine and mercaptopurine are excreted intact. In the 24 hour period after administration up to 50% of the dose is excreted in the urine with 10% as the parent drug. There is no data concerning azathioprine excretion in breast milk. Pharmacology and toxicology: Mode of action: Toxicodynamics: The principal toxic effect of azathioprine is bone marrow depression manifested by leukopenia, macrocytic anemia, pancytopenia, and thrombocytopenia, which may result in prolongation of clotting time and eventual hemorrhage. Pharmacodynamics: The exact mechanism of immunosuppressive activity of azathioprine has not been determined. Azathioprine which is an antagonist to purine metabolism may inhibit RNA and DNA synthesis. The drug may also be incorporated into nucleic acids resulting in chromosome breaks, malfunctioning of the nucleic acids, or synthesis of fraudulent proteins. The drug may also inhibit coenzyme formation and functioning, thereby interfering with cellular metabolism. Mitosis may be inhibited by the drug. In patients who undergo renal transplantation, azathioprine suppresses hypersensitivities of the cell-mediated type and causes variable alterations in antibody production. Human data: Adults: Severe pancytopenia has been observed in about 1% of the patients. Children: Lymphopenia, decreased IgG and IgM concentrations, cytomegalovirus infection. Cytogenetic damage was observed in human lymphocytes in vitro. Acute myelogenous leukemia and solid tumours have occurred in patients with rheumatoid arthritis who received the drug. Mutagenicity: Azathioprine is mutagenic in animals and humans, chromosomal abnormalities have been documented in humans receiving azathioprine, but the abnormalities were reversed following discontinuance of the drug. Interactions: Azathioprine dose should be reduced 75% when administered with allopurinol, as allopurinol affects the metabolism of mercaptopurine, a metabolite of azathioprine. Azathioprine may reduce the effect of certain neuromuscular blocking agents including curare and related non-depolarizing drugs. Certain cytotoxic agents may be additive or synergistic in producing toxicity when used in conjunction with azathioprine. The Committee on Safety of Medicines have advised that azathioprine and penicillamine should not be used concurrently. The effects of azathioprine and corticosteroids could be synergistic. Azathioprine may reduce the anticoagulant effect of warfarin. ANIMAL/PLANT STUDIES: Studies with animals have shown that the haemopoietic system is affected by azathioprine with depression of granulopoiesis, megakaryocytes and, hence, platelet formation. Reversible hepatoxicity has been observed in dogs. Various teratogenic effects have been observed in rabbits, showing skeletal abnormalities. In mice embryolethalite was observed. Carcinogenicity: Azathioprine is carcinogenic in animals. Teratogenicity: Azathioprine is teratogenic in rabbits and mice when given in dosages equivalent to the human dosage. Abnormalities included skeletal malformations and visceral anomalies. Mutagenicity: Azathioprine is mutagenic in the Ames test. Azathioprine antagonizes purine metabolism and may inhibit synthesis of DNA, RNA, and proteins. It may also interfere with cellular metabolism and inhibit mitosis. Its mechanism of action is likely due to incorporation of thiopurine analogues into the DNA structure, causing chain termination and cytotoxicity. Toxicity Data The oral LD50 for single doses of azathioprine in mice and rats are 2500 mg/kg and 400 mg/kg, respectively. Interactions Xanthine oxidase, an enzyme of major importance in the catabolism of metabolites of azathioprine, is blocked by allopurinol. If azathioprine and allopurinol are used in the same patient, the azathioprine dose must be decreased to 25 to 33% of the usual dose, but it is best not to use these two drugs together. Adverse effects resulting from coadministration of azathioprine with other myelosuppressive agents or ACE inhibitors include leukopenia, thrombocytopenia, and/or anemia ... Allopurinol inhibits the principal metabolic pathway of azathioprine, the oxidative metabolism of mercaptopurine by xanthine oxidase. This may lead to toxic accumulation of azathioprine with concomitant bone marrow depression. Therapeutic use may lead to bone marrow depression, hepatic dysfunction infection, drug fever, rash, urticarial eruption, hypersensitivity vasculitis, nausea, vomiting, and diarrhea, and possibly an increase in non-Hodgkin's lymphoma when used with corticosteroids in rheumatoid arthritis. Allopurinol-induced inhibition of xanthine oxidase-mediated metabolism may result in greatly increased azathioprine activity and toxicity; concurrent use should be avoided if possible, especially in renal transplant patients, because of the high risk of 6-mercaptopurine (azathioprine metabolite) accumulation and consequent azathioprine toxicity if the transplanted kidney is rejected; if concurrent use is essential, it is recommended that azathioprine dosage be reduced to one quarter to one third of the usual dosage, the patient be carefully monitored, and subsequent dosage adjustments be based on patient response and evidence of toxicity. For more Interactions (Complete) data for AZATHIOPRINE (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 535 mg/kg LD50 Rat intraperitoneal 300 mg/kg LD50 Rat intraduodenal 630 mg/kg LD50 Mouse oral 1389 mg/kg For more Non-Human Toxicity Values (Complete) data for AZATHIOPRINE (7 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Azathioprine also is indicated in the treatment of other immunological diseases including regional and ulcerative colitis, biliary cirrhosis, systemic dermatomyositis (polymyositis), glomerulonephritis, chronic active hepatitis, systemic lupus erythematosus (SLE), inflammatory myopathy, myasthenia gravis, nephrotic syndrome, pemphigus and pemphigoid. /NOT included in US product labeling/ Azathioprine is indicated for the management of severe, active, and erosive rheumatoid arthritis unresponsive to rest or conventional medications. /Included in US product labeling/ It /azathioprine/ is also also indicated in the prevention of rejection in cardiac, hepatic, and pancreatic transplantation. /NOT included in US product labeling/ Azathioprine is indicated as an adjunct for prevention of rejection in renal homotransplantation. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for AZATHIOPRINE (12 total), please visit the HSDB record page. Drug Warnings Azathioprine is a toxic drug and must be used only under close medical supervision. Other immunosuppressive therapy given concomitantly with azathioprine therapy may increase the toxic potential of the drug. Azathioprine may also cause rash, infection, drug fever, serum sickness, alopecia, arthralgia, retinopathy, Raynaud's disease, and pulmonary edema. Some of these adverse effects can occur as manifestations of rare hypersensitivity reactions. Azathioprine-induced hypersensitivity reactions are often characterized by a combination of symptoms, including fever, rigors, musculuskeletal symptoms (arthralgias, myalgias), and/or cutaneous effects (generalized erythematous or maculopapular rash with nonspecific inflammatory changes demonstrated on biopsy); pulmonary manifestations (eg, cough and/or dyspnea) and hypotension (which may be severe and, in the presence of fever, mimic septic shock) may also occur. Hepatotoxicity manifested by increased serum alkaline phosphatase, bilirubin, and/or aminotransferase concentrations may occur in patients receiving azathioprine, principally in allograft recipients. Azathioprine-induced hepatotoxicity following transplantation occurs most frequently within 6 months of transplantation and is generally reversible following discontinuance of the drug. Rare, but life-threatening hepatic veno-occlusive disease has occurred during chronic azathioprine therapy in several renal allograft recipients and in a patient with panuveitis; serious complications, including progressive portal hypertension, progressive liver failure requiring a portacaval shunt, progressive chronic liver failure with portal hypertension and esophageal varices, and/or rapid deterioration resulting in death, occurred in most of these patients. Veno-occlusive disease was associated with cytomegalovirus infection in some of these patients and with use of azathioprine but not with dosage of the drug, type or duration of renal allograft, or type of underlying renal disease. Reports to date suggest that the onset of hepatic veno-occlusive disease generally occurs after 1-2 years of therapy and that the disease occur principally in males. The clinical syndrome is usually manifested initially by jaundice, often followed by the development of ascites and other signs of partal hypertension. Serum alkaline phosphatase and bilirubin concentrations are usually elevated. Prognosis is poor. Because hepatic veno-occlusive disease may result in rapid clincial deterioration, prompt diagnosis and therapeutic intervention are necessary. Many clinicians suggest that liver biopsy to diagnose veno-occlusive disease should be performed in renal allograft recipients receiving azathioprine at the first sign of mild hepatic dysfunction. If veno-occlusive disease is evident, azathioprine therapy should be promptly and permanently discontinued; alternative immunosuppressive therapy should be considered and, if liver failure is progressive anticoagulation, a partacaval shunt, or hepatic allotransplantation should be considered. Hepatotoxicity occurs in less than 1% of patients with rheumatoid arthritis who receive azathioprine. Nausea, vomiting, anorexia, and diarrhea may occur in patients receiving large doses of azathioprine. Adverse GI effects may be minimized by giving the drug in divided doses and/or after meals. Vomiting with abdominal pain may occur rarely with a hypersensitivity pancreatitis. A GI hypersensitivity reaction characterized by severe nausea and vomiting has been reported. This reaction also may be accompanied by diarrhea, rash, fever, malaise, myalgias, elevations in liver enzymes, and, occasionally, hypotension. Symptoms of GI toxicity most often develop within the first several weeks of azathioprine therapy and are reversible upon discontinuance of the drug. The reaction can occur within several hours after rechallange with a single dose of the drug. Other adverse GI effects include ulceration of the mucous membranes of the mouth, esophagitis with possible ulceration, and steatorrhea. For more Drug Warnings (Complete) data for AZATHIOPRINE (33 total), please visit the HSDB record page. Pharmacodynamics Azathioprine is an immunosuppressive agent which functions through modulation of rac1 to induce T cell apoptosis, as well as other unknown immunosuppressive functions. It has a long duration of action as it is given daily, and has a narrow therapeutic index. Patients should be counselled regarding the risk of malignancies of the skin and lymphomas. |
| 分子式 |
C9H7N7O2S
|
|---|---|
| 分子量 |
277.26
|
| 精确质量 |
277.038
|
| 元素分析 |
C, 38.99; H, 2.54; N, 35.36; O, 11.54; S, 11.56
|
| CAS号 |
446-86-6
|
| 相关CAS号 |
Azathioprine-d3;2702733-53-5;Azathioprine sodium;55774-33-9;Azathioprine-13C4;1346600-71-2
|
| PubChem CID |
2265
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.9±0.1 g/cm3
|
| 沸点 |
685.7±55.0 °C at 760 mmHg
|
| 熔点 |
243-244°C
|
| 闪点 |
368.5±31.5 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.924
|
| LogP |
0.67
|
| tPSA |
143.4
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
354
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1C2=C(N=C([H])N=1)N=C([H])N2[H])C1=C([N+](=O)[O-])N=C([H])N1C([H])([H])[H]
|
| InChi Key |
LMEKQMALGUDUQG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H7N7O2S/c1-15-4-14-7(16(17)18)9(15)19-8-5-6(11-2-10-5)12-3-13-8/h2-4H,1H3,(H,10,11,12,13)
|
| 化学名 |
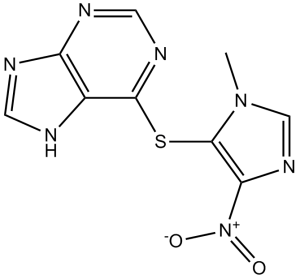
6-(3-methyl-5-nitroimidazol-4-yl)sulfanyl-7H-purine
|
| 别名 |
BW57-322; BW-57-322; Azathioprine; BW 57-322; BW 57 322; trade name: Imuran; Azasan; Imurel. Abbreviations: AZA; AZTP
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6067 mL | 18.0336 mL | 36.0672 mL | |
| 5 mM | 0.7213 mL | 3.6067 mL | 7.2134 mL | |
| 10 mM | 0.3607 mL | 1.8034 mL | 3.6067 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Evaluate the Safety and Efficacy of Satralizumab in Participants With Neuromyelitis Optica Spectrum Disorder (NMOSD)
CTID: NCT04660539
Phase: Phase 3 Status: Completed
Date: 2024-06-17