| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Rac GTPase (IC50 = 50 μM)
NSC 23766 mainly targets Rac1 GTPase, inhibiting Tiam1 (a guanine nucleotide exchange factor for Rac1)-Rac1 interaction to block Rac1 activation; the IC50 value for human recombinant Rac1 is 100 nM [1] NSC 23766 has no obvious inhibitory effect on other Rho family GTPases (Cdc42, RhoA) (IC50>1000 nM), showing high target specificity [2] |
|---|---|
| 体外研究 (In Vitro) |
NSC23766 经鉴定适合 Rac1 的表面凹槽,已知该凹槽对 GEF 规范至关重要。 NSC23766 以剂量依赖性方式有效抑制 Rac 特异性 GEF Trio 或 Tiam1 的结合和激活,而不干扰密切相关的 Cdc42 或 RhoA 各自 GEF 的结合或激活,也不干扰 Rac1 与 BcrGAP 或效应器 PAK1 的相互作用。 NSC 23766 积极调节细胞骨架上的 Rac GTPase 功能和许多细胞功能,包括细胞周期、细胞生长、粘附、迁移和基因转录。 NSC 23766 (50 μM) 有效阻断血清或血小板衍生生长因子诱导的 Rac1 激活和板状伪足形成,而不影响 NIH 3T3 细胞中内源性 Cdc42 或 RhoA 的活性。 NSC 23766 减少 Trio 或 Tiam1,但不减少 Vav、Lbc、Intersectin 或组成型活性 Rac1 突变体刺激的 NIH 3T3 细胞生长,并抑制 Trio、Tiam1 或 Ras 诱导的细胞转化。 NSC23766 剂量依赖性地抑制 PC-3 细胞增殖和贴壁依赖性生长。 25 μM NSC23766 可抑制 PC-3 细胞通过 Matrigel 的侵袭 85%。 [1] 50 μM NSC 23766 抑制凝血酶诱导的人血小板中 Rac1 和 Rac2 的激活以及血小板聚集。 NSC23766 可阻止 swAPP-HEK293 细胞中 Aβ40 和 Aβ42 的产生,而不影响 Notch 和 sAPPα。 NSC23766 可阻止细胞中的 γ-分泌酶活性,但不能作为直接的 γ-分泌酶抑制剂。 NSC23766 剂量依赖性地降低分泌型和细胞内 Aβ40 的水平,IC50 为 48.94 μM。 50 μM NSC 23766 可抑制 Aβ42 的释放 57.97%。 NSC23766 调节内皮一氧化氮合酶表达和内皮功能。 100 μM NSC23766 在牛主动脉 EC 中抑制 eNOS 启动子活性 60%,在 bEND.3 细胞中抑制 30% 至 35%。 NSC23766 抑制 Rac1 会破坏 eNOS mRNA 的稳定性,并将其半衰期缩短至 17 小时。 NSC23766 剂量依赖性地减弱 ACh 诱导的野生型小鼠主动脉环松弛。 NSC23766 抑制细胞生长并诱导细胞凋亡。 NSC23766 以剂量依赖性方式降低 MDA-MB-468 和 MDA-MB-231 细胞活力,IC50 约为 10 μM,与雌激素受体 (ER)、孕激素受体 (PR)、Her2、和p53突变。 NSC23766对MCF12A正常乳腺上皮细胞的存活影响很小。暴露于 NSC 23766 24 小时后,MDA-MB-231 细胞的 G1 期细胞数量从 41% 增加至 65%,同时 S 期和 G2-M 期细胞数量减少。 100 μM NSC23766 诱导凋亡 MDA-MB-468 增加六倍。 NSC23766 对乳腺癌细胞细胞周期停滞或凋亡的抑制作用是通过下调细胞周期蛋白 D1、生存素和 X 连锁蛋白凋亡抑制剂介导的。激酶测定:细胞在 10 cm 培养皿中以对数期生长,并在 0.5% 血清培养基中饥饿或以其他方式指示 24 小时,然后在含有 20 mM Tris HCl (pH 7.6)、100 mM NaCl、10 的缓冲液中裂解。 mM MgCl2、1% Nonidet P-40、10% 甘油和 1× 蛋白酶抑制剂混合物。澄清裂解物,标准化蛋白质浓度,并通过效应域下拉测定法测量裂解物中 GTP 结合的 Rac1。对于 His6-PAK1 PBD Pull-down 测定,细胞裂解物与从大肠杆菌中纯化的 Ni2+-琼脂糖固定的 His6-PAK1 PBD 结构域(每个约 1 μg)一起孵育 30 分钟。 Ni2+-琼脂糖共沉淀物在洗涤缓冲液中洗涤两次,并使用抗 Rac1 单克隆抗体进行免疫印迹分析。细胞测定:将细胞(1.5 × 104/mL 人乳腺癌细胞 MDA-MB-468)接种到含有 200 μL 培养基的 96 孔组织培养板的每个孔中。铺板 24 小时后,用 200 μL 含有所示浓度 NSC23766 的新鲜培养基更换培养基。处理结束时,每孔加入20μL MTS溶液,37℃孵育2小时。在 96 孔板读数器上读取 490 nm 处的吸光度。
NSC 23766 浓度依赖性抑制Rac1 GTP酶活性:10 μM浓度下,小鼠巨噬细胞中Rac1活性降低65%;50 μM浓度下,猪卵母细胞中Rac1活性降低78%,且不影响Cdc42、RhoA活性 [2][4] NSC 23766 抑制猪卵母细胞成熟:100 μM浓度处理44小时,第一极体排出率从72%降至28%,同时下调Cyclin B1表达(降低55%)、上调p21表达(升高2.3倍)[2] NSC 23766 影响小鼠原始卵泡形成:50 μM浓度处理卵巢颗粒细胞24小时,STAT3磷酸化水平降低60%,Jagged1、GDF9、BMP15的mRNA表达分别降低58%、52%、49% [3] NSC 23766 抑制炎症相关因子分泌:50 μM浓度处理LPS刺激的巨噬细胞,TNF-α、IL-6分泌量分别降低62%、57%,NF-κB p65核转位减少55% [4] NSC 23766 调节免疫细胞功能:50 μM浓度处理小鼠脾淋巴细胞,CD4+ T细胞增殖率从68%降至32%,IFN-γ分泌减少65%,IL-4分泌升高2.1倍 [1] |
| 体内研究 (In Vivo) |
NSC23766 诱导造血干细胞/祖细胞的动员。将 NSC23766 (2.5 mg/kg) 腹膜内施用到“活动性差”的 C57Bl/6 小鼠品系中,导致注射后 6 小时循环造血干细胞祖细胞增加两倍。 NSC23766 可减轻脂多糖诱导的小鼠急性肺损伤。 NSC23766以1或3mg/kg治疗不仅减少炎症细胞浸润和MPO活性,而且抑制促炎介质、肿瘤坏死因子-α和白介素-1β的mRNA表达。 NSC23766 还可以减少 LPS 挑战的肺部中伊文思蓝和白蛋白的积累。
NSC 23766 以50 mg/kg剂量腹腔注射,每周3次,持续12周,可显著预防NOD小鼠1型糖尿病发生:发病率从85%降至30%,胰腺胰岛炎评分从3.2分降至1.1分,胰岛CD4+ T细胞浸润减少70% [1] NSC 23766 腹腔注射30 mg/kg,可缓解蜜蜂毒液诱导的小鼠急性炎症痛:给药后2小时,机械痛阈从0.8 g升至2.5 g,热痛阈从12.5秒升至21.3秒,脊髓组织中Rac1活性降低68% [4] NSC 23766 以20 mg/kg腹腔注射,每日1次,持续7天,可减少新生小鼠卵巢原始卵泡数量:从对照组的126个/卵巢降至78个/卵巢,STAT3磷酸化水平降低52% [3] |
| 酶活实验 |
使用蛋白酶和磷酸酶抑制剂匀浆来自腰椎增大的新鲜脊髓组织,然后使用缓冲液裂解组织。收集上清液,并在 4°C 下以 12,000×g 离心 5 分钟后,与 PAK-PBD 珠子在 4°C 下在旋转器上孵育 1 小时。然后以 5000×g 离心 3 分钟,将珠子沉淀。在 4°C 下。在 LaemmLi 缓冲液中重悬后,将所得沉淀煮沸两分钟。对珠子样品进行蛋白质印迹分析。蛋白质印迹分析还用于确定每个样品中的总 Rac1。
采用GST-pull-down法检测Rac1活性:提取细胞或组织总蛋白,与GST-PAK1-PBD融合蛋白(特异性结合活化态Rac1-GTP)孵育,4℃孵育1小时后,洗涤沉淀并进行Western blot检测,定量分析活化态Rac1的相对含量,计算NSC 23766对Rac1活性的抑制率 [2] 采用荧光共振能量转移(FRET)法验证结合特异性:将重组Rac1与荧光标记GTP混合,加入梯度浓度的NSC 23766,37℃孵育30分钟,检测FRET信号变化,计算药物与Rac1的结合亲和力及IC50值 [4] |
| 细胞实验 |
96 孔组织培养板每孔接种 1.5 × 10 4 /mL 细胞和 200 μL 培养基。 24 小时电镀期后,用 200 μL 含有指定浓度 NSC23766 的新培养基替换培养基。处理期结束后,将 20 μL MTS 溶液引入每个孔中,并在 37°C 下孵育两小时。使用 96 孔板读数器测量 490 nm 处的吸光度。
猪卵母细胞分离自卵巢,接种于含M199培养基的培养皿,38.5℃、5%CO2培养2小时后,加入梯度浓度的NSC 23766(10、50、100 μM),继续培养44小时;观察第一极体排出情况,提取蛋白通过Western blot检测Rac1、Cdc42、RhoA活性及Cyclin B1、p21表达 [2] 小鼠卵巢颗粒细胞分离后接种于24孔板(1×10⁵个/孔),DMEM/F12培养基培养24小时,加入NSC 23766(20、50、100 μM)孵育24小时;提取总RNA,qPCR检测Jagged1、GDF9、BMP15的mRNA表达;提取蛋白检测STAT3磷酸化水平 [3] 小鼠巨噬细胞接种于96孔板(5×10⁴个/孔),培养16小时后,加入LPS(1 μg/mL)刺激,同步加入NSC 23766(10、50 μM),孵育24小时;ELISA法检测细胞上清中TNF-α、IL-6浓度,GST-pull-down法检测Rac1活性 [4] |
| 动物实验 |
At 7 weeks of age, Balb/c control and NOD mice are split into four groups (n=8/group). At eight weeks of age, two experimental groups—Balb/c and NOD mice—receive NSC23766 (2.5 mg/kg/day, i.p./daily), while the other two groups—control Balb/c and NOD mice—receive an equal volume of saline. Throughout 34 weeks, weekly blood glucose and body weight measurements are made.
Two groups of experimental animals (Balb/c and NOD mice) received NSC23766, while the two control groups received equal volume of saline. Body weights and blood glucose were measured every week for 34 weeks. Rac1 activation in pancreatic islets was measured by GLISA activation assay. Rac1 and CHOP expression was determined by Western Blotting.[1]
NOD mice (female, 4 weeks old) were randomly divided into groups: the administration group was given NSC 23766 dissolved in 5% DMSO+95% normal saline at 50 mg/kg intraperitoneally three times a week for 12 weeks; the control group was given an equal volume of solvent. Blood glucose was detected every 2 weeks (≥11.1 mmol/L was diagnosed as diabetes), and pancreatic tissue was collected at the end of the experiment for HE staining and immunohistochemistry to analyze insulitis severity and T cell infiltration [1] ICR mice (male, 6 weeks old) were used to establish a bee venom-induced acute inflammatory pain model: bee venom (20 μL/mouse) was subcutaneously injected into the right hind paw, and drugs were administered 30 minutes later; NSC 23766 was dissolved in normal saline and injected intraperitoneally at 10, 30 mg/kg. Mechanical pain threshold (von Frey filament method) and thermal pain threshold (hot plate method) were detected at 1, 2, and 4 hours after administration, and Rac1 activity in spinal cord and dorsal root ganglia was detected at the end of the experiment [4] Neonatal C57BL/6 mice (1 day after birth) were randomly divided into groups: the administration group was given NSC 23766 20 mg/kg intraperitoneally once daily for 7 days; the control group was given normal saline. Ovarian tissue was collected at the end of the experiment, primordial follicle number was counted by HE staining, and STAT3 phosphorylation was detected by Western blot [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
NSC 23766 shows no obvious cytotoxicity with cell survival rate ≥80% at in vitro concentration ≤100 μM [2][3]
When NSC 23766 was administered intraperitoneally at 50 mg/kg (three times a week for 12 weeks), NOD mice showed normal weight gain, no statistical difference in serum ALT, AST, BUN, Cr levels compared with the control group, and no obvious pathological damage to pancreas, liver, or kidney [1] The maximum tolerated dose of NSC 23766 for a single intraperitoneal injection is ≥100 mg/kg in mice, with no acute toxicity observed [4] |
| 参考文献 |
|
| 其他信息 |
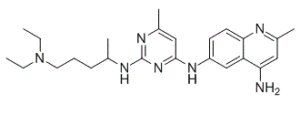
NSC 23766 is an aminopyrimidine that is 6-methylpyrimidine-2,4-diamine in which the amino groups at positions 2 and 4 are substituted by 5-(diethylamino)pentan-2-yl and 4-amino-2-methylquinolin-6-yl groups respectively. An inhibitor of the signalling G-protein known as RAC1 (Ras-related C3 botulinum toxin substrate 1). It has a role as an EC 3.6.5.2 (small monomeric GTPase) inhibitor, an antiviral agent, a muscarinic antagonist and an apoptosis inducer. It is an aminoquinoline, an aminopyrimidine, a primary amino compound, a secondary amino compound and a tertiary amino compound.
Background/aims: Type 1 diabetes (T1D) is characterized by absolute insulin deficiency due to destruction of pancreatic β-cells by cytokines (e.g., interleukin-1β; IL-1β) released by invading immune cells. The mechanisms by which these cytokines induce β-cell dysfunction remain poorly understood. Recent evidence suggests that excessive generation of reactive oxygen species (ROS) by the phagocyte-like NADPH oxidase2 (Nox2), along with significantly low levels of antioxidants in β-cells, drive them toward oxidative damage. Rac1, a small G-protein, is one of the members of Nox2 holoenzyme. We recently reported that NSC23766, a known inhibitor of Rac1, significantly attenuated cytokine-induced Nox2 activation and ROS generation in pancreatic islet β-cells in vitro. Herein, we determined the effects of NSC23766 (2.5 mg/kg/day, i.p/daily) on the development of diabetes in the NOD mouse, a model for T1D. Methods: Two groups of experimental animals (Balb/c and NOD mice) received NSC23766, while the two control groups received equal volume of saline. Body weights and blood glucose were measured every week for 34 weeks. Rac1 activation in pancreatic islets was measured by GLISA activation assay. Rac1 and CHOP expression was determined by Western Blotting. Results: Our findings indicate that administration of NSC23766 significantly prevented the development of spontaneous diabetes in the NOD mice. Furthermore, NSC23766 markedly suppressed Rac1 expression and activity and the endoplasmic reticulum stress (CHOP expression) in NOD islets. Conclusions: Our findings provide the first evidence implicating the role of Tiam1-Rac1-Nox2 signaling pathway in the onset of spontaneous diabetes in the NOD mouse model.[1] Mammalian oocyte asymmetric division relies on the eccentric positioning of the spindle, resulting in the polar body formation. Small signaling G protein Rac1 is a member of GTPases, which regulates a diverse array of cellular events, including the control of cell growth, cytoskeletal reorganization, and the activation of protein kinases. However, effects of Rac1 on the porcine oocyte maturation and early embryo development are not fully understood. In present study we investigated the role of Rac1 in oocyte maturation and embryo cleavage. We first found that Rac1 localized at the cortex of the porcine oocytes, and disrupting the Rac1 activities by treating with NSC 23766 led to the failure of polar body emission. In addition, a majority of treated oocytes exhibited abnormal spindle morphology, indicating that Rac1 may involve into porcine oocyte spindle formation. This might be due to the regulation of Rac1 on MAPK, since p-MAPK expression decreased after NSC 23766 treatments. Moreover, we found that the position of most meiotic spindles in treated oocytes were away from the cortex, indicating the roles of Rac1 on meiotic spindle positioning. Our results also showed that inhibition of Rac1 activity caused the failure of early embryo development. Therefore, our study showed the critical roles of Rac1 GTPase on porcine oocyte maturation and early embryo cleavage.[2] NSC 23766 is the first specific inhibitor of the Tiam1-Rac1 signaling pathway, which inhibits Rac1 activation by blocking Tiam1-Rac1 interaction, thereby regulating immune response, inflammatory signals, cell cycle, and germ cell development [1] Preclinical studies have shown that NSC 23766 has potential value in the treatment of autoimmune diseases (type 1 diabetes) and acute inflammatory pain, and can regulate ovarian primordial follicle formation, serving as a tool drug for reproduction-related research [1][3][4] The mechanism of NSC 23766 involves multiple downstream signaling pathways, including STAT3, NF-κB, and cell cycle regulatory pathways, with pleiotropy but high target specificity [2][3][4] |
| 分子式 |
C24H35N7
|
|
|---|---|---|
| 分子量 |
421.58
|
|
| 精确质量 |
421.29539415
|
|
| CAS号 |
733767-34-5
|
|
| 相关CAS号 |
NSC 23766 trihydrochloride;1177865-17-6
|
|
| PubChem CID |
409805
|
|
| 外观&性状 |
Off-white to light yellow solid powder
|
|
| 密度 |
1.16g/cm3
|
|
| 沸点 |
632.4ºC at 760 mmHg
|
|
| 闪点 |
336.2ºC
|
|
| 折射率 |
1.646
|
|
| LogP |
8.023
|
|
| tPSA |
91.99
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
514
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
NC1=CC(C)=NC2=CC=C(NC3=NC(NC(C)CCCN(CC)CC)=NC(C)=C3)C=C12
|
|
| InChi Key |
DEFBCZWQLILOJF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H35N7/c1-6-31(7-2)12-8-9-16(3)27-24-28-18(5)14-23(30-24)29-19-10-11-22-20(15-19)21(25)13-17(4)26-22/h10-11,13-16H,6-9,12H2,1-5H3,(H2,25,26)(H2,27,28,29,30)
|
|
| 化学名 |
6-N-[2-[5-(diethylamino)pentan-2-ylamino]-6-methylpyrimidin-4-yl]-2-methylquinoline-4,6-diamine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3720 mL | 11.8601 mL | 23.7203 mL | |
| 5 mM | 0.4744 mL | 2.3720 mL | 4.7441 mL | |
| 10 mM | 0.2372 mL | 1.1860 mL | 2.3720 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|