| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HIF-1α(hypoxia-inducible factor-1α)
BAY 87-2243 is a selective inhibitor of mitochondrial complex I (NADH:ubiquinone oxidoreductase), with an IC50 of 1.8 nM in purified bovine heart mitochondria. It shows no significant inhibition of other mitochondrial respiratory chain complexes (complex II, III, IV) even at concentrations up to 10 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
对于体外研究,将BAY 87-2243制备为10mmol/L的二甲基亚砜(DMSO)储备溶液,并在相关测定介质中稀释。 BAY 87-2243 抑制荧光素酶活性,IC50 值约为 0.7 nM。 BAY 87-2243 在 HCT116luc 细胞的蛋白质水平上抑制 HIF 靶基因 CA9 的缺氧表达,IC50 值约为 2 nM。根据氧敏感荧光染料 LUX-MitoXpress[1] 的测定,BAY 87-2243 的 IC50 值小于 10 nM,可抑制线粒体耗氧量。 BAY-87-2243 抑制核 HIF-1α 蛋白表达。与相对血管面积 (RVA) 和灌注血管 (PF) 相比,BAY-87-2243 给药约 18 天可显着降低 HIF-1α 蛋白表达、坏死分数 (NF)(平均 9% vs. 35.6%,p =0.0002) 和哌莫硝唑缺氧分数 (pHF)(平均 2.4% (BAY-87-2243) 对比 17.6%(载体),p<0.0001)[2]。
跨癌细胞系抗增殖活性:BAY 87-2243 在常氧(21% O₂)和缺氧(1% O₂)条件下对多种人癌细胞系产生剂量依赖性生长抑制。72小时MTT实验IC50值:A549(肺癌,常氧12 nM/缺氧8 nM)、HCT116(结直肠癌,常氧15 nM/缺氧9 nM)、SK-MEL-28(黑色素瘤,常氧10 nM/缺氧7 nM)、PC-3(前列腺癌,常氧18 nM/缺氧11 nM)。缺氧条件下细胞对BAY 87-2243 的敏感性提高约1.3–1.6倍[1] - 缺氧诱导基因表达抑制:A549细胞暴露于缺氧环境(1% O₂,24小时)时,BAY 87-2243(5–20 nM)剂量依赖性降低缺氧诱导因子-1α(HIF-1α)蛋白表达(抑制40–85%)及其靶基因(血管内皮生长因子VEGF,mRNA下调55–90%;葡萄糖转运体1 GLUT1,mRNA下调45–80%),检测方法为蛋白质印迹法和qPCR[1] - 线粒体功能破坏:BAY 87-2243(10–30 nM)处理HCT116细胞16小时后,线粒体ATP生成减少50–75%(荧光素酶法),活性氧(ROS)水平升高2.5–4倍(DCFH-DA染色),线粒体膜电位(ΔΨm)降低30–60%(JC-1染色),提示线粒体功能障碍[1] - 与分次放疗的协同作用:在头颈部鳞癌细胞(FaDu)中,BAY 87-2243(3–10 nM)联合分次放疗(2 Gy/次,每周5次,共3周)较单独放疗降低细胞存活。5 nM药物联合10 Gy放疗时,存活分数(SF)为0.08(联合组)vs 0.25(单独放疗组),放射增敏比(RSR)为1.6[2] |
| 体内研究 (In Vivo) |
将 H460 细胞皮下注射到裸鼠体内,一旦肿瘤形成,小鼠每天接受 BAY 87-2243(0.5、1、2 和 4 mg/kg)口服灌胃治疗,持续三周。根据 HIF-1 靶基因 CA9、ANGPTL4 和 EGLN3 mRNA 表达水平的剂量依赖性降低,BAY 87-2243 降低了肿瘤重量。然而,体内化合物治疗对缺氧不敏感的EGLN2基因或HIF-1α本身的mRNA表达水平没有影响[1]。
异种移植模型单药抗肿瘤活性:对携带皮下肿瘤(A549、HCT116、SK-MEL-28)的雌性裸鼠(6–8周龄),给予BAY 87-2243(10 mg/kg,灌胃,每日1次)处理21天。肿瘤生长抑制率(TGI):A549(72%,治疗组体积290 mm³ vs 溶剂组1030 mm³,P<0.01)、HCT116(68%,治疗组320 mm³ vs 溶剂组1000 mm³,P<0.01)、SK-MEL-28(75%,治疗组260 mm³ vs 溶剂组1040 mm³,P<0.01)。在A549原位肺癌模型中,BAY 87-2243(10 mg/kg,口服)将中位生存期从28天(溶剂组)延长至45天(P<0.001)[1] - 头颈部肿瘤异种移植与放疗联合活性:对携带皮下FaDu/CAL27(头颈部鳞癌)肿瘤的雄性BALB/c裸鼠(7周龄),分为4组:溶剂组、BAY 87-2243 单药组(3 mg/kg,灌胃,每日1次)、单独放疗组(2 Gy/次,每周5次,共3周)、联合组。FaDu肿瘤:联合组肿瘤生长延迟(TGD)28天,显著长于单独放疗组(8天)和单药组(5天),局部控制率(LCR)60%(单独放疗组10%,P<0.01);CAL27肿瘤:联合组TGD 25天,长于单独放疗组(7天)和单药组(4天),LCR 55%(单独放疗组8%,P<0.01)[2] |
| 酶活实验 |
脯氨酸羟化酶活性测定[1]
试验化合物对脯氨酰羟化酶2(PHD2)活性的影响如前所述进行测定15,并进行了一些修改:从Sf9细胞裂解物中纯化重组人PHD2,并用于包被在NeutrAvidin板上的生物素化HIF-1α556-574肽的羟基化。在与用铕标记的纯化的Von Hippel-Lindau L-Elongin-B-Elongin-Complex复合物孵育并加入增强剂溶液后,通过用Tecan infinite M200平板读取器测量时间分辨荧光来定量脱羧肽。 HIF-1α的蛋白质印迹分析[2] 根据先前所述的既定方案进行一次蛋白质印迹[24]。根据制造商的说明,使用NE-PER细胞核和细胞质试剂盒制备蛋白质样品。使用的抗体是小鼠单克隆抗人HIF-1α(1:250)和兔多克隆抗组蛋白-H2B(1:500)和抗钙蛋白酶1(1:100),分别作为核或细胞质细胞区室的负载对照。将核HIF-1α带强度标准化为组蛋白-H2B水平。 线粒体复合物I活性测定:通过差速离心纯化牛心线粒体,实验在含0.2 mM NADH(底物)和0.05 mM辅酶Q1(电子受体)的反应缓冲液(25 mM KH₂PO₄ pH 7.4、5 mM MgCl₂、0.2 mM EDTA)中进行。加入系列浓度的BAY 87-2243(0.1–10 nM),加入线粒体(0.1 mg蛋白/mL)启动反应,37°C下每30秒检测340 nm处吸光度(反映NADH氧化),持续5分钟。复合物I活性以NADH氧化速率(μmol/min/mg蛋白)计算,通过四参数逻辑模型拟合活性抑制曲线得到IC50值[1] |
| 细胞实验 |
细胞测定[1]
为了高通量筛选由~830000种化合物组成的小分子文库,用含有荧光素酶报告系统的载体稳定转染HCT-116细胞,该报告系统四次偶联到来自人血管内皮生长因子(VEGF)启动子的HRE(HCT 116-4xVEGF-Luc)。将细胞以3×10E4细胞/孔铺板并孵育过夜,然后加入测试化合物(在DMSO中为5mmol/L),并将板置于1%pO2的缺氧室中16小时。结果以从常氧、未处理的对照中减去基线水平后的任意单位的发光计数给出。为了测量细胞复合体I的活性,将H1299细胞与编码毒蚁幼虫点击甲虫萤光素酶的pcDNA3载体共转染。亚克隆显示高发光和剂量依赖性鱼藤酮敏感性的克隆(H1299tluc),然后通过发光测量用于进一步深入分析细胞复合物I的活性。简言之,将H1299tluc细胞(1500/孔)接种到白色384孔板中。在不含葡萄糖但补充有11 mmol/L半乳糖的Dulbecco改良鹰培养基(DMEM)中培养2天后,向每个孔中加入10μL荧光素/抑制剂混合物(150μmol/L d-荧光素,在Tyrode中终浓度为0.4%的DMSO),并在37°C下孵育1小时。使用内部开发的平板阅读器进行发光测量。测量后,加入20μL琥珀酸酯(0.67mol/L,蒂罗尔中的pH 5.3,最终浓度25mmol/L)。然后在进行第二次测量之前,将板在室温下再孵育1小时。通过使用PiggyBac转座子介导的基因转移11,在巨细胞病毒(CMV)启动子和C末端HA标签的控制下用编码NDI1的pcDNA3载体转染,产生表达来自酿酒酵母(NDI1)的NADH-Q-氧化还原酶的H1299tluc细胞。通过在含有11.2mmol/L葡萄糖的DMEM培养基中在20nmol/L鱼藤酮存在下培养来进行阳性克隆的选择。如上所述使用具有高发光的鱼藤酮不敏感克隆。萤光素酶活性以DMSO处理的细胞的%表示。为了评估BAY 87-2243的细胞毒性,将各细胞系的2.000个细胞接种在96孔板中,并在含有10%FCS的适当生长培养基中培养。在接种48小时后24小时加入不同浓度的BAY 87-2243,并使用细胞滴度发光测定法测定细胞活力 CA9蛋白的定量。[1] 将HCT 116-4xVEGF-Luc细胞以3×10E4细胞/孔的速度接种在96孔板中,并在37°C下在含有5%CO2的湿润培养箱中在正常氧气水平下孵育过夜,然后在不存在或存在各种浓度的BAY 87-2243的情况下改变缺氧条件(1%pO2,24小时)。使用MN/CAIX酶联免疫吸附测定法定量细胞裂解物中HIF靶基因碳酸酐酶9(CA9)的蛋白质表达水平。 MTT抗增殖实验:将癌细胞(A549、HCT116、SK-MEL-28)以5×10³细胞/孔接种于96孔板,常氧(21% O₂)或缺氧(1% O₂、5% CO₂、94% N₂)条件下过夜孵育。加入BAY 87-2243(0.1–100 nM),培养72小时。每孔加入10 μL MTT试剂(5 mg/mL),继续孵育4小时,150 μL DMSO溶解甲臜结晶,检测570 nm处吸光度。细胞存活率(%)=(处理组吸光度/对照组吸光度)×100,通过GraphPad Prism计算IC50[1] - HIF-1α/VEGF蛋白质印迹实验:A549细胞在含BAY 87-2243(5–20 nM)的缺氧环境(1% O₂)中培养24小时,用含蛋白酶抑制剂的RIPA缓冲液裂解,30 μg蛋白进行10% SDS-PAGE电泳后转移至PVDF膜。膜用5%脱脂牛奶室温封闭1小时,4°C下与抗HIF-1α、抗VEGF一抗孵育过夜,再与HRP标记二抗室温孵育1小时。ECL显色后,ImageJ定量条带强度[1] - 克隆形成存活实验(放射敏感性):FaDu/CAL27细胞以200–1000细胞/孔接种于6孔板,过夜孵育。放疗前1小时加入BAY 87-2243(3–10 nM),给予0–10 Gy单次放疗。培养14天后,甲醇固定克隆,结晶紫染色并计数(>50细胞为克隆)。存活分数(SF)=(克隆数×接种效率)/接种细胞数,10 Gy时放射增敏比(RSR)=单独放疗SF/联合治疗SF[2] |
| 动物实验 |
In vivo tumor study [1]
For in vivo studies, BAY 87-2243 was formulated in a 1% (v/v) solution of ethanol/solutol/water (10/40/50%). Animals were given BAY 87-2243 (0.5, 1, 2, and 4 mg/kg) or vehicle control once daily by oral gavage. Tumor xenograft experiment was carried out on female immune-deficient, athymic NMRI nude mice, aged 7–9 weeks, weighing 20–25 g in full accordance with the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Marketing and Education issued by the New York Academy of Sciences' Ad Hoc Committee on Animal Research. The lung carcinoma xenograft mouse model was established by subcutaneous injection into the right flank with 0.1 mL H460 tumor cells (1.5 × 10E6) mixed 1:1 with Matrigel. Mice were randomized into control and treatment groups when tumors reached a size of more than 40 mm2. Body weight was monitored as a measure for treatment-related, acute toxicity. Tumor area (measured by caliper) or tumor weight (measured when mice were sacrificed 21 days after cell injection) was calculated by the formula 100−100 × (tumor weight/area of treatment group)/(tumor weight/area of vehicle group). Murine plasma pharmacokinetic analyses[1] Plasma concentrations of unchanged BAY 87-2243 were determined by liquid chromatography coupled to a tandem mass spectrometer (LC-MS/MS). Briefly, murine plasma was centrifuged and subsequently precipitated by addition of acetonitrile and an internal standard. The supernatants were subjected to high-performance LC connected to a MS/MS (API 3000, Applied Biosystems, Darmstadt, Germany) via a Turbo Ion Spray interface. Tumor sampling[2] BAY-87-2243 was dissolved in carrier solution (10% ethanol, 40% Solutol® HS15, 50% sterile distilled water) and administered orally by gavage (9 mg/kg/body weight [b.w.]). When UT-SCC-5 hSCC xenografts in nude mice reached 6 mm in diameter BAY-87-2243 or carrier was administered before and/or during RT or radiochemotherapy with concomitant cisplatin (RCT). Local tumor control was evaluated 150 days after irradiation and the doses to control 50% of tumors (TCD50) were compared between treatment arms. Tumors were excised at different time points during BAY-87-2243 or carrier treatment for western blot and immunohistological investigations. Tumor monotherapy protocol (A549/HCT116/SK-MEL-28): Female nude mice (6–8 weeks old) were subcutaneously injected with 5×10⁶ cancer cells (100 μL PBS/matrigel, 1:1) into the right flank. When tumors reached ~100 mm³, mice were grouped (n=6/group): vehicle (0.5% methylcellulose in PBS, oral, daily) and BAY 87-2243 (10 mg/kg, dissolved in 0.5% methylcellulose, oral, daily). Treatment lasted 21 days. Tumor volume (length × width² / 2) was measured every 3 days, body weight weekly. For orthotopic A549 tumors: 2×10⁶ cells were injected into the left lung, treatment started 7 days post-injection, and survival was monitored [1] - Radiation combination protocol (FaDu/CAL27): Male BALB/c nude mice (7 weeks old) were subcutaneously injected with 4×10⁶ FaDu/CAL27 cells (100 μL PBS/matrigel, 1:1). When tumors reached ~120 mm³, mice were grouped (n=5/group): (1) vehicle (0.5% methylcellulose, oral, daily); (2) BAY 87-2243 (3 mg/kg, oral, daily); (3) irradiation (2 Gy/fraction, 5 fractions/week for 3 weeks, delivered to tumors via X-ray); (4) combination (drug 1 h before irradiation). Tumor volume was measured every 2 days, and local tumor control (no tumor regrowth for 60 days) was assessed [2] |
| 药代性质 (ADME/PK) |
Oral absorption and PK in rats: Male Sprague-Dawley rats (250–300 g) received BAY 87-2243 via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg). Oral bioavailability was 65%. For oral administration: Cmax = 85 ng/mL (Tmax = 1.2 h), terminal t1/2 = 4.8 h, AUC0-24h = 420 ng·h/mL. For intravenous administration: Cmax = 220 ng/mL, t1/2 = 4.5 h, AUC0-∞ = 510 ng·h/mL [1]
- Plasma protein binding: In human plasma, BAY 87-2243 had a protein binding rate of 98%, primarily to albumin and α1-acid glycoprotein (measured by equilibrium dialysis) [1] - Tumor penetration: In A549 xenograft mice, oral BAY 87-2243 (10 mg/kg) resulted in tumor tissue concentrations of 12 nM at 2 h post-administration, which was ~1.5-fold higher than plasma concentrations (8 nM) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Repeat-dose toxicity in rats: Male/female Sprague-Dawley rats (n=4/sex/group) received BAY 87-2243 (5, 15, 30 mg/kg, oral, daily) for 28 days. No mortality was observed. The no-observed-adverse-effect level (NOAEL) was 15 mg/kg. At 30 mg/kg: mild weight loss (~7%), increased serum ALT (2.1-fold vs. control) and AST (1.8-fold vs. control), and mild hepatocellular vacuolation (histopathology); no renal toxicity [1]
- Toxicity in xenograft mice: In all tumor models, BAY 87-2243 (3–10 mg/kg, oral, daily) caused ≤5% body weight loss, with no signs of overt toxicity (e.g., lethargy, diarrhea). Combination with irradiation did not increase toxicity compared to single agents [1,2] |
| 参考文献 |
|
| 其他信息 |
The activation of the transcription factor hypoxia-inducible factor-1 (HIF-1) plays an essential role in tumor development, tumor progression, and resistance to chemo- and radiotherapy. In order to identify compounds targeting the HIF pathway, a small molecule library was screened using a luciferase-driven HIF-1 reporter cell line under hypoxia. The high-throughput screening led to the identification of a class of aminoalkyl-substituted compounds that inhibited hypoxia-induced HIF-1 target gene expression in human lung cancer cell lines at low nanomolar concentrations. Lead structure BAY 87-2243 was found to inhibit HIF-1α and HIF-2α protein accumulation under hypoxic conditions in non-small cell lung cancer (NSCLC) cell line H460 but had no effect on HIF-1α protein levels induced by the hypoxia mimetics desferrioxamine or cobalt chloride. BAY 87-2243 had no effect on HIF target gene expression levels in RCC4 cells lacking Von Hippel-Lindau (VHL) activity nor did the compound affect the activity of HIF prolyl hydroxylase-2. Antitumor activity of BAY 87-2243, suppression of HIF-1α protein levels, and reduction of HIF-1 target gene expression in vivo were demonstrated in a H460 xenograft model. BAY 87-2243 did not inhibit cell proliferation under standard conditions. However under glucose depletion, a condition favoring mitochondrial ATP generation as energy source, BAY 87-2243 inhibited cell proliferation in the nanomolar range. Further experiments revealed that BAY 87-2243 inhibits mitochondrial complex I activity but has no effect on complex III activity. Interference with mitochondrial function to reduce hypoxia-induced HIF-1 activity in tumors might be an interesting therapeutic approach to overcome chemo- and radiotherapy-resistance of hypoxic tumors[1].
Mechanism of action: BAY 87-2243 exerts antitumor effects by two key mechanisms: (1) Inhibiting mitochondrial complex I, reducing ATP production and increasing ROS, which suppresses hypoxia-inducible factor-1α (HIF-1α) stabilization (HIF-1α requires ATP for translation); (2) Downregulating HIF-1α target genes (VEGF, GLUT1) that promote angiogenesis and glycolysis, critical for hypoxic tumor survival. When combined with irradiation, it enhances DNA damage by reducing tumor hypoxia (via inhibiting angiogenesis) and impairing DNA repair [1,2] - Preclinical development focus: BAY 87-2243 was evaluated preclinically for solid tumors with high hypoxia (e.g., lung, colorectal, melanoma, head and neck cancer), particularly as a radiosensitizer to improve local tumor control in radiotherapy-resistant hypoxic tumors [1,2] |
| 分子式 |
C26H26F3N7O2
|
|---|---|
| 分子量 |
525.5256
|
| 精确质量 |
525.21
|
| 元素分析 |
C, 59.42; H, 4.99; F, 10.85; N, 18.66; O, 6.09
|
| CAS号 |
1227158-85-1
|
| 相关CAS号 |
1227158-85-1
|
| PubChem CID |
67377767
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
677.7±65.0 °C at 760 mmHg
|
| 闪点 |
363.7±34.3 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.674
|
| LogP |
4.2
|
| tPSA |
85.34
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
774
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
CDJNNOJINJAXPV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C26H26F3N7O2/c1-17-14-22(25-31-24(33-38-25)19-2-6-21(7-3-19)37-26(27,28)29)32-36(17)16-18-8-9-30-23(15-18)35-12-10-34(11-13-35)20-4-5-20/h2-3,6-9,14-15,20H,4-5,10-13,16H2,1H3
|
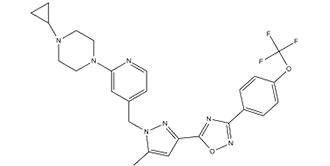
| 化学名 |
1-cyclopropyl-4-[4-[[5-methyl-3-[3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl]-1H-pyrazol-1-yl]methyl]-2-pyridinyl]-piperazine
|
| 别名 |
BAY-872243; BAY 872243; BAY-872243; BAY-87-2243; BAY87-2243; BAY 87-2243
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO:<1 mg/mL
Water:<1 mg/mL
Ethanol: 8 mg/mL(15.22 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.76 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.76 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9028 mL | 9.5142 mL | 19.0284 mL | |
| 5 mM | 0.3806 mL | 1.9028 mL | 3.8057 mL | |
| 10 mM | 0.1903 mL | 0.9514 mL | 1.9028 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01297530 | Terminated | Drug: BAY87-2243 | Neoplasms | Bayer | April 2011 | Phase 1 |
(A) Chemical structure of BAY 87-2243. (B) BAY 87-2243 suppresses hypoxia-induced reporter gene activity (left), and CA9 protein levels in vitro in HCT-116 cell lysates (right). Cancer Med. 2013 Oct;2(5):611-24. |
BAY 87-2243 is inactive in RCC4 cells lacking functional VHL protein or in H460 cells silenced for EGLN1.Cancer Med.2013 Oct;2(5):611-24. |
BAY 87-2243 reduces tumor weight, hypoxia-inducible factor (HIF)-1α protein levels, and HIF-1 target gene expression in H460 xenograft tumors. Cancer Med. 2013 Oct;2(5):611-24. |