| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
ATP-citrate lyase (ACL); AMPK
|
|---|---|
| 体外研究 (In Vitro) |
Bempedoic Acid (ETC-1002) 以 Ca2+/钙调蛋白依赖性激酶 β 独立和肝激酶 β 1 依赖性方式激活 AMP 激活的蛋白激酶,且腺苷酸能量电荷没有可检测到的变化。已证明,bempedoic 酸可快速转化转化为肝脏中的 CoA 硫酯,直接抑制 ATP-柠檬酸裂解酶[1]。 AMP 激活蛋白激酶 (AMPK) 磷酸化增加与经 Bempedoic 酸 (ETC-1002) 处理的细胞中 MAP 激酶活性降低以及促炎细胞因子和趋化因子产生减少相关[2]。
ETC-1002是一种研究药物,目前处于ii期开发,用于治疗血脂异常和其他心脏代谢危险因素。在血脂异常的受试者中,ETC-1002不仅可以降低血浆LDL胆固醇,还可以显著降低hsCRP水平,hsCRP是炎症的临床生物标志物。在原代人单核细胞源性巨噬细胞和体内炎症模型中进一步研究了ETC-1002的抗炎特性。在用ETC-1002处理的细胞中,amp活化的蛋白激酶(AMPK)磷酸化水平的增加与MAP激酶活性的降低和促炎细胞因子和趋化因子的产生减少相一致。通过sirna介导的巨噬细胞肝激酶B1 (LKB1)的沉默,显著消除了AMPK磷酸化和ETC-1002对可溶性炎症介质的抑制作用,表明ETC-1002激活AMPK并通过LKB1依赖机制发挥其抗炎作用。在体内,ETC-1002抑制巯基乙酸盐诱导的白细胞归巢到小鼠腹腔。同样,在饮食诱导的肥胖小鼠模型中,ETC-1002恢复了脂肪AMPK活性,降低了JNK磷酸化,并降低了巨噬细胞特异性标志物4F/80的表达。这些数据与附睾脂肪垫块减少和炎症脂肪组织释放白细胞介素(IL)-6一致。因此,ETC-1002可以通过减少与胰岛素抵抗和代谢综合征血管并发症相关的全身性炎症,为具有心脏代谢危险因素的患者提供进一步的临床益处。[2] |
| 体内研究 (In Vivo) |
Bempedoic Acid (ETC-1002) 治疗两周可导致大鼠肝脏中 AMPK 和 ACC 磷酸化显着且持久的增加。在大鼠肝脏中,Bempedoic 酸的含量比 CoA 硫酯高 100 倍,并且与 AMPK 激活有关[1]。 Bempedoic Acid (ETC-1002) 可抑制白细胞归巢至小鼠腹腔的能力。在饮食诱导肥胖的小鼠模型中,Bempedoic Acid 可改善脂肪 AMPK 活性,降低 JNK 磷酸化,并降低巨噬细胞特异性标记物 4F/80 的表达[2]。
ETC-1002(8-羟基-2,2,14,14-四甲基戊二酸)是一种用于治疗血脂异常和其他心脏代谢危险因素的新型研究药物。ETC-1002在临床前疾病模型中表现出的降血脂、抗动脉粥样硬化、抗肥胖和降血糖的特性被认为是由于双重抑制甾醇和脂肪酸合成以及增强线粒体长链脂肪酸β-氧化。然而,介导这些活性的分子机制仍未明确。本文描述的研究表明,ETC-1002游离酸以Ca(2+)/钙调素依赖性激酶β独立和肝激酶β 1依赖的方式激活amp活化的蛋白激酶,而腺苷酸能量电荷没有可检测到的变化。此外,ETC-1002在肝脏中迅速形成CoA硫酯,直接抑制atp -柠檬酸裂解酶。这些不同的分子机制在体外和体内对脂质和碳水化合物代谢的有益作用方面是互补的。与这些机制一致的是,在高脂血症仓鼠模型中,ETC-1002治疗降低了循环促动脉粥样硬化脂蛋白、肝脂和体重,并在饮食性肥胖小鼠模型中降低了体重并改善了血糖控制。ETC-1002有望作为一种新的治疗方法,改善与代谢综合征相关的多种危险因素,并使心血管疾病患者受益。[1] |
| 酶活实验 |
葡萄糖生成试验[1]
葡萄糖产量测定原代大鼠肝细胞培养。细胞在不含葡萄糖和酚红的DMEM中培养,其中含有10 mM乳酸,1 mM丙酮酸和非必需氨基酸(葡萄糖生产缓冲液,GPB)。为了评估ETC-1002对胰高血糖素刺激的葡萄糖生成的影响,将细胞与不同浓度的ETC-1002 (0.1 ~ 100 μM)的0.3 μM胰高血糖素孵育。随着时间的推移对媒体进行采样。在指定的处理后,细胞在GPB中洗涤两次。然后将细胞再孵育一个小时,通过添加不含ETC-1002的含有等量胰高血糖素浓度的GPB来评估葡萄糖的产生。细胞孵育1小时,使用葡萄糖氧化酶测定试剂盒测定培养基中葡萄糖的浓度。 ETC-1002配方/ ETC-1002- coa合成[2] 对于体外检测,ETC-1002在无菌二甲基亚砜(DMSO)中采用30和100 mM的无菌技术配制,并在无菌微离心管中在4°C下保存长达四周(稳定性评估)。ETC-1002在含12 mM HEPES、1万U/ml青霉素、100 μg/ml链霉素的无血清RPMI 1640中配制工作液。用描述的大鼠肝微粒体合成ETC-1002-CoA。 将7.5×化合物加入96孔PolyPlate中,每个孔含有60μL缓冲液,底物为CoA(200μM)、ATP(400μM)和[14C]柠檬酸盐。用4μL(300纳克/孔)ACL开始反应,并将平板在37°C下孵育3小时。 |
| 细胞实验 |
在原代大鼠肝细胞培养物中测量葡萄糖的产生。它含有非必需氨基酸、10 mM 乳酸、1 mM 丙酮酸,并且不含葡萄糖和酚红。细胞在该混合物中培养。 Bempedoic Acid(0.1 至 100 μM)与各种浓度的细胞一起孵育[1]。
蛋白质阵列[2] 在分化期结束时,用PBS洗涤巨噬细胞,切换到含有5%自体血清的RPMI 1640,并补充14 mM HEPES, 100 U/ml青霉素,50 U/ml链霉素和2 mM l -谷氨酰胺。ETC-1002在100 ng/ml来自大肠杆菌0111:B4的脂多糖(LPS)刺激前1 h加入不同浓度(50 μM和100 μM)的培养基中。在LPS刺激后12小时收集MDMs培养基,根据制造商的说明,使用Proteome Profiler Human Cytokine Array Kit, Panel A和Human Matrix Metalloproteinase Array进行检测。细胞因子和基质金属蛋白酶(MMP)阵列的数据被柯达4000MM图像工作站捕获和分析。每个分析物的净信号强度表示为每个单独阵列膜的内参标准的百分比。数据以平均值±SEM表示。组间比较采用单因素方差分析。采用Bonferroni事后多重比较检验评估方差分析显示的显著性差异。P≤0.05接受显著性。[2] |
| 动物实验 |
Rats: Male Wistar Han rats are fasted for 48 hours and then given a single dose of bempedoic acid before receiving a second 48-hour feeding of a high-carbohydrate diet. Rats are kept on a standard chow diet and given oral gavage doses of bempedoic acid for a two-week assessment. The dose is 30 mg/kg/day given in the morning. Food is discontinued two hours before the final oral dose of engine control or bempedoic acid after nutritional staging and/or dosing[1].
For in vivo experiments, ETC-1002 dosing solutions were formulated by preparing a disodium salt aqueous solution using 2:1 molar ratio of NaOH to ETC-1002 in water. Carboxymethyl cellulose (CMC) and Tween-20 were added to make a final solution containing 0.5% CMC and 0.025% Tween with a final pH 7–8. Compound concentrations in dosing solutions were administered at a volume of 10 ml/kg body.[1],2] Wistar rats.[1] Male Wistar Han [Crl:WI] rats weighing 225–250 g were acclimated to the laboratory environment for seven days, housed 2–3 per cage in a temperature controlled room, and maintained on a 12 h light and dark cycle with ad libitum access to food and water. Prior to single-dose ETC-1002 administration, rats were fasted for 48 h and refed a high-carbohydrate diet for an additional 48 h. For two-week assessment, rats were maintained on standard chow diet (Purina 5001) and dosed by oral gavage with ETC-1002 at 30 mg/kg/day for two weeks in the morning. Following nutritional staging and/or dosing, food was withdrawn 2 h prior to last the oral dose of vehicle control or ETC-1002. Blood and liver were collected from isofluorane-anesthetized animals 2 or 8 h after the last dose, blood was collected from the subclavian vein, and liver tissue was harvested by freeze clamp. The freeze-clamped liver samples were held frozen in liquid nitrogen immediately following excision and stored at −70°C. Plasma triglycerides, β-hydroxybutyrate (β-HBA), and total cholesterol levels were measured with commercially available kits (Wako Diagnostics, Richmond, VA) adapted to a 96-well format. Golden Syrian hamsters.[1] Male golden Syrian hamsters were obtained from Charles River (Montreal, QC) at 8–10 weeks of age and weighed 100–120 g. Animals were maintained on Prolab RMH 1000 standard rodent chow diet during a seven-day quarantine period. Following randomization into treatment groups (n = 6), hyperlipidemia was induced by feeding high-fat, high-cholesterol (HFHC) Prolab RMH 1000 diet containing: 11.5% coconut oil, 11.5% corn oil, 5% fructose, and 0.5% cholesterol. During the study, animals were individually housed in an environmentally controlled room with a 12 h light and dark cycle. Following two weeks on HFHC diet, hamsters were dosed by oral gavage once daily with vehicle (0.5% carboxymethyl cellulose and 0.025% Tween-20, pH 7–8) or vehicle plus ETC-1002 (30 mg/kg) for three weeks. Body weights were recorded every two days at the beginning of dosing, and food consumption was measured every four days. Blood samples were collected by administering isoflurane anesthesia and bleeding from the orbital venous plexus in lithium heparinized tubes during the study and by cardiac puncture under anesthesia at the end of the study. Plasma samples were analyzed for triglycerides, total cholesterol, nonesterified fatty acids, and β-hydroxybutyrate on an automated chemistry analyzer. Liver and epididymal fat were collected, weighed, frozen in liquid nitrogen, and stored at −80°C until processing. All hamster procedures were conducted in accordance with the current guidelines for animal welfare at the Hospital for Sick Children and were in compliance with National Institutes of Health Publication 86-23, 1985; Animal Welfare act, 1966, as amended in 1970, 1976, and 1985, 9 CFR Parts 1, 2, and 3. Diet-induced obesity in mice.[1] Male C57BL/6N mice were obtained from Taconic at 8 weeks of age and singly housed on α-dri paper bedding on a normal 12 h light and dark cycle (6 AM to 6 PM). Upon arrival mice, were fed a high-fat diet (HFD) containing 60% kcal fat for 12 weeks. Mice were randomized into two treatment arms at 20 weeks of age based on 4 h fasted blood glucose and body weight and received oral dosing of either CMC/Tween vehicle or 30 mg/kg/day ETC-1002 q.d in the morning for an additional two weeks. Body weight and food consumption were monitored throughout the study. Following the two-week dosing period, food was removed at 8 AM, and bedding was changed 2 h prior to oral administration of ETC-1002. Two hours post dose, fasting samples were collected. Fasting blood glucose levels were measured immediately prior to anesthesia using a hand-held Alphatrak glucometer (Abbott, Chicago, IL), with blood collected by unrestrained tail snip. For insulin determinations, blood was collected under isoflurane anesthesia via retro-orbital sinus into EDTA-coated tubes, and plasma was isolated by centrifugation. Plasma insulin levels were measured with a commercially available ELISA |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bempedoic acid is rapidly absorbed in the small intestine. The Tmax of the 180mg tablet is estimated at 3.5 hours. Bempedoic acid's conjugates are primarily eliminated via the urine (70%) and the feces (30%). A total of 5% of the unchanged drug is excreted in the urine and feces, combined. The apparent volume of distribution of bempedoic acid is about 18L. The clearance (CL/F) of bempedoic acid at steady state was estimated at 11.2 mL/min during clinical trials. Metabolism / Metabolites The two main metabolites of bempedoic metabolism are ETC-1002-CoA and ESP15228. Bempedoic acid is primarily eliminated via the metabolism of its acyl glucuronide. This drug is reversibly converted to an active metabolite (ESP15228) based on observations during in vitro studies. Both compounds resulting from the metabolism of bempedoic acid are metabolized to become inactive glucuronide conjugates by the enzyme UGT2B7. Biological Half-Life The half-life of bempedoic acid ranges between 15 and 24 hours. Prescribing information indicates a clearance of 21 hours +/- 11 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No relevant published information exists on the use of bempedoic acid during breastfeeding. Bempedoic acid and its metabolites are 99% plasma protein bound, so amounts in milk are likely very low. However, because of a concern with disruption of infant lipid metabolism, bempedoic acid is best avoided during breastfeeding. An alternate drug is preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The plasma protein binding of bempedoic acid and its metabolites is about 99%. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Bempedoic acid inhibits the synthesis of cholesterol in the liver, reducing LDL-C levels. This reduces the development of atherosclerotic plaques that may increase the risk of cardiovascular events. Earlier clinical trials studying the effects of bempedoic acid showed a dose‐dependent reduction of LDL‐C levels in addition to decreased LDL particle number, and reduced levels of apolipoprotein B, non–HDL cholesterol, and high‐sensitivity C‐reactive protein. Due to its unique mechanism of action, bempedoic acid is not associated with myositis, an adverse effect that frequently accompanies statin therapy. More recent trials have supported that this drug significantly decreases LDL-C levels after 12 weeks of therapy and provides additional lowering of LDL-C when combined with ezetimibe and statin therapy. The effects of bempedoic acid on mortality are currently unknown. |
| 分子式 |
C19H36O5
|
|---|---|
| 分子量 |
344.4861
|
| 精确质量 |
344.256
|
| 元素分析 |
C, 66.25; H, 10.53; O, 23.22
|
| CAS号 |
738606-46-7
|
| 相关CAS号 |
Bempedoic acid-d4;2408131-70-2;Bempedoic acid-d5;2408131-71-3
|
| PubChem CID |
10472693
|
| 外观&性状 |
White to light yellow solid powder
|
| 熔点 |
87-92
|
| LogP |
4.469
|
| tPSA |
94.83
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
351
|
| 定义原子立体中心数目 |
0
|
| SMILES |
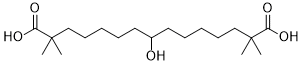
OC(CCCCCC(C(=O)O)(C)C)CCCCCC(C(=O)O)(C)C
|
| InChi Key |
HYHMLYSLQUKXKP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H36O5/c1-18(2,16(21)22)13-9-5-7-11-15(20)12-8-6-10-14-19(3,4)17(23)24/h15,20H,5-14H2,1-4H3,(H,21,22)(H,23,24)
|
| 化学名 |
8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid
|
| 别名 |
ETC-1002; ETC 1002; ETC1002; ESP-55016; Bempedoate; 8-Hydroxy-2,2,14,14-tetramethylpentadecanedioic acid; Nexletol; Nilemdo; ESP-55016; Bempedoic acid; ETC-1002ESP55016; ETC1002ESP
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~290.3 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.87 mg/mL (8.33 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.87 mg/mL (8.33 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.26 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (7.26 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 25.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (7.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 6 中的溶解度: 0.57 mg/mL (1.65 mM) in 1% DMSO + 99% Saline (这些助溶剂从左到右依次添加,逐一添加),悬浮液;澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9028 mL | 14.5142 mL | 29.0284 mL | |
| 5 mM | 0.5806 mL | 2.9028 mL | 5.8057 mL | |
| 10 mM | 0.2903 mL | 1.4514 mL | 2.9028 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Randomized, Double-blind, Placebo-controlled Study to Assess the Effects of Bempedoic Acid (ETC-1002) on the Occurrence of Major Cardiovascular Events in Patients with, or at high risk for, Cardiovascular Disease who are Statin Intolerant.
CTID: null
Phase: Phase 3 Status: Ongoing, GB - no longer in EU/EEA, Completed
Date: 2017-02-16