AICA ribonucleotide; AICA riboside; AICAR; Acadesine; AICA Riboside; ARA100; ARA-100; 2627-69-2; AICA-riboside; Arasine; AICA riboside; AIC-Riboside; Acadesina; ARA 100; GP 1 110; SCH-900395; SCH 900395; SCH900395; AICAR
阿卡地新;5-氨基-1-beta-D-呋喃核糖基-1H-咪唑-4-甲酰胺;5-氨基咪唑-4-甲酰胺-1-B-D-呋喃核糖苷;5-氨基咪唑-4-甲酰胺-1-β-D-呋喃核糖苷;5-氨基咪唑-4-甲酰胺核苷酸转甲酰酶;阿卡地辛;阿卡地新 Acadesine;阿卡地新(AICAR)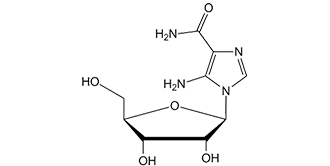
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
AMPK; Autophagy; Mitophagy; Human Endogenous Metabolite
The primary target of Acadesine (AICAR; NSC-105823) is AMP-activated protein kinase (AMPK), a central regulator of cellular energy homeostasis. - In [1]: It activates rat liver AMPK with a half-maximal effective concentration (EC50) of ~100 μM, and shows no inhibitory activity against protein kinase A (PKA) or protein kinase C (PKC) (IC50 > 1 mM) [1] - In [2]: In human acute myeloid leukemia HL-60 cells, it activates AMPK with an EC50 of ~80 μM, and exhibits no cross-reactivity with Janus kinase 2 (JAK2) or other hematopoietic kinases (IC50 > 500 μM) [2] - In [3]: For human hepatocyte AMPK complexes, the EC50 is ~120 μM; it does not affect the PI3K-Akt signaling pathway (IC50 > 200 μM) [3] |
|---|---|
| 体外研究 (In Vitro) |
Acadesine (500 μM) 经过长达 30-40 分钟的处理后,可增加分离肝细胞提取物中的 ZMP 含量,然后保持相当恒定在约 4 nmol/g。 Acadesine (500 μM) 在 15 分钟内导致大鼠肝细胞中 AMPK 瞬时激活 12 倍,以及脂肪细胞中 AMPK 瞬时激活 2-3 倍,而不影响 ATP、ADP 或 AMP 水平。 Acadesine (500 μM) 会显着抑制大鼠肝细胞中脂肪酸和甾醇的合成。 Acadesine (500 μM) 还会导致 HMG-CoA 还原酶急剧失活。[1] cadesine 的 EC50 为 380 M,以剂量依赖性方式诱导 B-CLL 细胞凋亡。阿卡地辛 (0.5 mM) 将 20 名代表性患者的 B-CLL 细胞活力从 68% 降低至 26%。 Caspase 激活和线粒体细胞色素 c 释放由阿卡地辛 (acadesine) (0.5 mM) 引起。必须摄取 Acadesine (0.5 mM) 并磷酸化,才能引起 B-CLL 细胞凋亡并激活 AMPK。 Acadesine (0.5 mM) 显着降低 B 细胞的活力,但不降低 T 细胞的活力,对 B-CLL 患者的 T 细胞的活力影响极小。 [2] 在 K562、LAMA-84 和 JURL-MK1 细胞中,杜松碱会导致细胞代谢丧失。它还可以有效杀死具有 T315I-BCR-ABL 突变的伊马替尼耐药 K562 细胞和 Ba/F3 细胞。由于 GF109203X 和 Ro-32-0432(经典和新 PKC 的抑制剂)都会抵消 Accadesine 的作用,因此 Accadesine 会导致 K562 细胞中几种 PKC 同工型的运动和激活。第 10 天,阿卡地辛以剂量依赖性方式抑制 K562 集落形成。其生长抑制作用在浓度为 0.25 mM 时就已经很明显,并且在 2.5 mM 时达到最大。 [3] Accadesine 在体外以浓度依赖性方式降低 LPS 刺激的中性粒细胞上 CD18 的表达。 [4] 血液中的 cadesine (1 mM) 可显着抑制由 N-甲酰基-甲硫氨酰-亮氨酰-苯丙氨酸引起的粒细胞 CD11b 上调(平均 61%)。 [5]
1. 调控肝脏糖代谢(来自[1]): - 原代大鼠肝细胞用Acadesine(50 μM、100 μM、200 μM)处理24小时,浓度依赖性抑制胰高血糖素诱导的葡萄糖生成:100 μM时抑制率约40%,200 μM时达65%(葡萄糖氧化酶法检测)。Western blot显示AMPK下游底物乙酰辅酶A羧化酶(ACC)Ser79位点磷酸化水平(p-ACC/ACC比值)较对照升高2.5倍[1] 2. 白血病细胞抗增殖活性(来自[2]): - 人HL-60白血病细胞用Acadesine(25 μM、50 μM、100 μM)处理72小时,细胞活力(MTT法)呈剂量依赖性降低,IC50约为60 μM。流式细胞术显示G2/M期细胞周期阻滞:G2/M期细胞比例从对照的15%升至100 μM处理组的42%。Western blot显示细胞周期抑制剂p21表达上调3.1倍[2] 3. 促进骨骼肌脂肪酸氧化(来自[3]): - 人骨骼肌成肌细胞分化为肌管后,用Acadesine(100 μM、200 μM)处理16小时,脂肪酸氧化率([14C]-棕榈酸掺入法)较对照升高~30%(100 μM)和~55%(200 μM)。实时定量PCR(qPCR)显示200 μM时脂肪酸转运蛋白1(FATP1)mRNA表达上调1.8倍[3] |
| 体内研究 (In Vivo) |
在 K562 细胞异种移植小鼠模型中,cadesine (50 mg/kg) 显着降低肿瘤的发展。 [3] 当施用卡地辛 (10 mg/kg) 时,猪的血流动力学稳定性需要更多的液体。猪死腔通气、恒定潮气量时的峰值吸气压以及 LPS 诱导的肺毛细血管蛋白通透性均受到 cadesine (10 mg/kg) 的抑制。 [4]
1. 白血病异种移植模型的抗肿瘤疗效(来自[2]): - 6~8周龄BALB/c裸鼠右侧胁腹皮下接种HL-60细胞(5×10⁶个细胞/只)。当肿瘤体积达~100 mm³时,小鼠随机分为3组(每组6只):溶剂组(0.9%生理盐水,腹腔注射)、Acadesine 50 mg/kg组(腹腔注射,每日1次)、Acadesine 100 mg/kg组(腹腔注射,每日1次)。治疗21天后,100 mg/kg组肿瘤体积减少~50%(380±45 mm³ vs 溶剂组760±62 mm³),中位生存期延长~30%(35天 vs 溶剂组27天)[2] 2. 高脂饮食(HFD)小鼠代谢紊乱改善(来自[3]): - C57BL/6小鼠高脂饮食(60%脂肪)12周,诱导胰岛素抵抗和肝脂肪变性。随后用Acadesine(150 mg/kg,溶于5% DMSO+95%生理盐水,口服灌胃,每日1次)或溶剂处理14天。Acadesine组空腹血糖(FBG)较溶剂组降低~25%(7.2±0.5 mmol/L vs 9.6±0.8 mmol/L),肝脏甘油三酯含量减少~40%(脂质提取试剂盒检测)[3] |
| 酶活实验 |
在半固体甲基纤维素培养基中,向 K562 细胞系或原代细胞(103 CD34+ 细胞/mL)给予阿卡地辛。细胞系和原代 CD34+ 细胞分别用 MethoCult H4100 或 H4236 培养。培养 10 天后,通过添加 1 mg/mL 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物 (MTT) 试剂发现菌落,并使用 Image J 对它们进行评分量化软件。
AMP活化蛋白激酶(AMPK)被认为通过关闭生物合成途径来保护细胞免受环境胁迫(如热休克),关键信号是AMP的升高。通过开发一种在完整细胞中激活激酶的特异性试剂,可以促进激酶级联新靶点的鉴定。用5-氨基咪唑-4-甲酰胺核糖核苷(AICAR)孵育大鼠肝细胞会导致单磷酰化衍生物(5-氨基咪唑4-甲酰胺核糖核苷酸;ZMP)在细胞内积累。ZMP模拟AMP对AMPK的激活作用,即直接变构激活和AMPK激酶促进磷酸化。与在完整细胞中激活AMPK的现有方法(如果糖、热休克)不同,AICAR不会干扰ATP、ADP或AMP的细胞含量。用AICAR孵育肝细胞会激活AMPK,因为磷酸化增加,导致AMPK的已知靶标(3-羟基-3-甲基戊二酰辅酶a还原酶)磷酸化和失活,并且几乎完全停止了两种已知的靶标途径,即脂肪酸和甾醇合成。用AICAR孵育分离的脂肪细胞可以拮抗异丙肾上腺素诱导的脂肪分解。这提供了直接证据,表明AMPK对环AMP依赖性蛋白激酶激活激素敏感脂肪酶的抑制作用,以前在无细胞检测中得到证实,也在完整细胞中起作用。AICAR应该是识别由蛋白激酶级联调控的新靶途径和过程的有用工具[1]。 1. 大鼠肝脏AMPK激活实验(来自[1]): - 试剂制备:制备Sf9昆虫细胞表达并经亲和层析纯化的重组大鼠肝脏AMPK;将AMPK特异性底物肽(AMARA肽,序列:AMARAASAAALARRR)溶于反应缓冲液(50 mM Tris-HCl pH7.4、10 mM MgCl₂、1 mM二硫苏糖醇(DTT)),终浓度200 μM;将[γ-³²P]ATP稀释至10 μM,比活度~2000 cpm/pmol[1] - 实验设置:Acadesine用DMSO系列稀释为25 μM、50 μM、100 μM、200 μM、400 μM,加入反应混合物(DMSO终浓度≤1%)。反应混合物含反应缓冲液、AMARA肽和[γ-³²P]ATP。加入重组AMPK(终浓度20 nM)启动反应,30°C孵育30分钟。设置溶剂对照(仅DMSO)和阳性对照(5 mM AMP),每组3个技术重复[1] - 检测与分析:取25 μL反应混合物点样到P81磷酸纤维素滤纸上,用1%磷酸洗涤3次(每次5分钟)去除未结合的[γ-³²P]ATP,丙酮漂洗后风干。液体闪烁计数测放射性,计算相对于溶剂组的AMPK激活倍数,四参数逻辑模型拟合EC50[1] 2. 激酶选择性实验(来自[2]): - 试剂制备:纯化重组人JAK2、PKA、PKCα;为每种激酶优化反应缓冲液(如JAK2缓冲液含50 mM HEPES pH7.5、10 mM MgCl₂、0.1 mM Na₃VO₄)[2] - 实验设置:Acadesine(100 μM、200 μM、500 μM、1000 μM)与各激酶(10 nM)及其特异性底物(如JAK2底物肽:EPQpYEEIPIYEPG)在37°C孵育40分钟,通过ADP-Glo™实验(检测ADP生成)测定激酶活性[2] - 分析:计算相对于溶剂组的抑制率,浓度达500 μM时对所有检测激酶的抑制率均<10%,证实对AMPK的选择性[2] |
| 细胞实验 |
将 HepG2 细胞(5×105 个细胞)接种到 6 孔培养板中,然后在转染前在无血清培养基中培养 12 小时。 FuGENE6转染试剂用于转染一微克质粒。转染5小时后,除去培养基,然后将添加或不添加AICAR (0.1-1.0 mM)的培养基添加到每个孔中。每 24 小时更换一次刺激介质[1]。
Acadesine/阿卡德新,5-氨基咪唑-4-甲酰胺(AICA)核糖苷,在所有测试样本中诱导B细胞慢性淋巴细胞白血病(B-CLL)细胞凋亡(n=70)。B-CLL细胞的半最大有效浓度(EC(50))为380+/-60微M(n=5)。半胱氨酸天冬氨酸蛋白酶抑制剂Z-VAD.fmk完全阻断了阿卡德辛诱导的细胞凋亡,这涉及半胱氨酸天冬氨酰蛋白酶-3、-8和-9的激活以及细胞色素c的释放。用阿卡德辛孵育B-CLL细胞诱导了腺苷一磷酸活化蛋白激酶(AMPK)的磷酸化,表明它被阿卡德辛激活。核苷转运抑制剂硝基苄硫肌苷(NBTI)、腺苷激酶抑制剂5-碘胸苷和腺苷完全抑制了阿卡塞辛诱导的细胞凋亡和AMPK磷酸化,表明阿卡塞辛掺入细胞及其随后磷酸化为AICA核糖肽(ZMP)是诱导细胞凋亡所必需的。蛋白激酶A和丝裂原活化蛋白激酶的抑制剂并不能保护B-CLL细胞免受阿卡德辛诱导的凋亡。此外,阿卡德新对p53水平或磷酸化没有影响,表明凋亡触发中存在p53非依赖性机制。正常B淋巴细胞与B-CLL细胞对阿卡德新诱导的凋亡一样敏感。然而,在高达4 mM的剂量下,B-CLL患者的T细胞仅受到阿卡德新的轻微影响。用阿卡德新处理的T细胞中没有发生AMPK磷酸化。当B-CLL细胞和T细胞都用0.5 mM阿卡德辛处理时,细胞内ZMP水平高于T细胞,这表明ZMP的积累是激活AMPK和诱导凋亡所必需的。这些结果表明,AMPK参与了B-CLL细胞凋亡控制的新途径,并提高了在B-CLL治疗中使用阿卡德新的可能性[2]。 1. 原代大鼠肝细胞葡萄糖生成实验(来自[1]): - 细胞制备:胶原酶灌注大鼠肝脏分离原代肝细胞,以1×10⁵个细胞/孔接种到24孔板,含10%胎牛血清(FBS)的DMEM培养基中37°C、5% CO₂孵育过夜[1] - 药物处理:更换培养基为含Acadesine(50 μM、100 μM、200 μM)和胰高血糖素(10 nM)的无糖DMEM,孵育24小时,收集培养上清用于葡萄糖检测[1] - 检测:葡萄糖氧化酶试剂盒测上清葡萄糖浓度;RIPA缓冲液提取细胞总蛋白,BCA法定量蛋白用于标准化。胰高血糖素诱导的葡萄糖生成抑制率按[(胰高血糖素组葡萄糖生成量 - Acadesine组葡萄糖生成量)/胰高血糖素组葡萄糖生成量]×100%计算[1] 2. HL-60细胞活力与细胞周期实验(来自[2]): - 活力实验:HL-60细胞以5×10³个细胞/孔接种到96孔板,含10% FBS的RPMI-1640培养基培养24小时。更换培养基为含Acadesine(25 μM、50 μM、100 μM)的培养基,孵育72小时。加入20 μL MTT溶液(5 mg/mL,溶于PBS),继续孵育4小时。吸去上清,加入150 μL DMSO溶解甲瓒结晶,测570 nm吸光度,逻辑回归计算IC50[2] - 细胞周期实验:HL-60细胞以2×10⁵个细胞/孔接种到6孔板,100 μM Acadesine处理48小时,4°C 70%乙醇固定过夜。用碘化丙啶(PI,50 μg/mL)和RNase A(100 μg/mL)37°C染色30分钟,流式细胞术分析,流式软件计算G0/G1、S、G2/M期细胞比例[2] 3. 人骨骼肌肌管脂肪酸氧化实验(来自[3]): - 细胞制备:人骨骼肌成肌细胞在含10% FBS的DMEM中培养至80%汇合度,更换为含2%马血清的DMEM诱导分化为肌管(7天),以5×10⁴个细胞/孔接种到12孔板[3] - 药物处理:Acadesine(100 μM、200 μM)加入培养基,肌管孵育16小时。加入[14C]-棕榈酸(0.5 μCi/孔)继续孵育2小时,孔内插入含CO₂捕获液(2 M NaOH)的小室收集氧化的[14C]-棕榈酸[3] - 检测:将CO₂捕获液转移至闪烁瓶,液体闪烁计数测放射性,按肌管总蛋白含量(BCA法)标准化脂肪酸氧化率[3] |
| 动物实验 |
Mice: Fourteen-week-old lean (Lepob/+ or Lepob/+) and ob/ob (Lepob/Lepob) male mice are uesd. After the 14-day experimental treatment (24 h after AICAR injection, including a 12-h fast), the plantar flexor complex muscle is cleanly (tendon-to-tendon) excised from an anesthetized mouse. The muscle is rapidly weighed, followed by histology processing or freezing in liquid nitrogen and storing at -80°C. Following a direct needle puncture into the heart to collect blood, the anesthetized mice are killed by transection of the diaphragm and removal of the entire heart. Subcutaneous injections of AICAR or saline (control) are made into the lateral distal region of the back. AICAR is given orally once daily for 14 days at a dose of 0.5 mg/g. Injections of saline (control) are performed in a manner and at volumes identical to those used for AICAR treatment. Prior to death, a person's weight is measured.
Rats: Male ZDF rats aged 5 weeks received a single subcutaneous injection of AICAR (0.5 mg/g body weight) or underwent a single bout of treadmill running (60 minutes, speed of 25 m/min at 5% incline). Controls (n=5 in each group) are untreated ZDF rats. Rats are killed by cervical dislocation one hour following subcutaneous AICAR injection or right away following treadmill use. Red and white gastrocnemius muscles are immediately removed to prevent the effects of muscle spasm and hypoxia, and they are then immediately freeze clamped to measure the AMPK activity later. 1. HL-60 xenograft experiment (from [2]): - Animal housing: BALB/c nude mice (6–8 weeks old, male) were housed in a specific pathogen-free (SPF) environment with a 12-hour light/dark cycle, constant temperature (22±2°C), and constant humidity (50±5%). Mice had free access to standard rodent chow and sterile water [2] - Tumor inoculation: HL-60 cells were cultured to logarithmic growth phase, trypsinized, and resuspended in PBS at a concentration of 5×10⁷ cells/mL. Each mouse was subcutaneously injected with 0.1 mL of the cell suspension (5×10⁶ cells) into the right flank [2] - Grouping and dosing: When tumors grew to an average volume of ~100 mm³, mice were randomly divided into 3 groups (n=6/group): (1) Vehicle group: 0.9% normal saline, 0.2 mL/mouse, intraperitoneal injection, once daily; (2) Low-dose group: Acadesine 50 mg/kg, dissolved in 0.9% normal saline, 0.2 mL/mouse, intraperitoneal injection, once daily; (3) High-dose group: Acadesine 100 mg/kg, same solvent and administration route/frequency as the low-dose group. Treatment lasted for 21 days [2] - Monitoring and sampling: Tumor volume (calculated as length × width² / 2) and body weight were measured every 3 days. Mice were monitored for survival until the endpoint (tumor volume > 1500 mm³ or severe morbidity). At the endpoint, tumors were excised for histological analysis (H&E staining) [2] 2. HFD-fed mouse metabolic experiment (from [3]): - Animal model: C57BL/6 mice (6 weeks old, male) were fed a HFD (60% fat) for 12 weeks to induce metabolic disorders. Mice were fasted for 12 hours before the experiment to measure baseline FBG [3] - Drug formulation and dosing: Acadesine was dissolved in 5% DMSO + 95% normal saline to a concentration of 15 mg/mL. Mice were divided into 2 groups (n=6/group): (1) Vehicle group: 5% DMSO + 95% normal saline, 10 mL/kg, oral gavage, once daily; (2) Acadesine group: 150 mg/kg, 10 mL/kg, oral gavage, once daily. Treatment lasted for 14 days [3] - Efficacy assessment: FBG was measured via tail vein blood using a glucose meter every 3 days. At the end of treatment, mice were euthanized, and liver tissue was collected to measure triglyceride content via a lipid extraction kit [3] |
| 药代性质 (ADME/PK) |
Biological Half-Life
1 week 1. Rat pharmacokinetic parameters (from [3]): - Study design: Male Sprague-Dawley (SD) rats (250–300 g) were divided into 2 groups (n=4/group): oral administration (150 mg/kg) and intravenous injection (50 mg/kg) of Acadesine [3] - Sample collection: Blood samples were collected from the jugular vein at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 12 hours post-dosing. Plasma was separated via centrifugation (3000×g, 10 minutes at 4°C) [3] - Key parameters: (1) Oral bioavailability: ~40%; (2) Half-life (t1/2): ~2.8 hours (oral) and ~1.9 hours (intravenous); (3) Peak concentration (Cmax): ~2.5 μg/mL (oral, reached at 1 hour); (4) Area under the curve (AUC₀-∞): ~19.2 μg·h/mL (oral) and ~24.5 μg·h/mL (intravenous) [3] 2. Metabolic profile (from [1]): - In rat liver microsomes, Acadesine (100 μM) was metabolized to its active form, 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP), with a conversion rate of ~85% after 2 hours of incubation. No other major metabolites were detected via HPLC analysis [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Negligible (approximately 1%) 1. Acute toxicity in mice (from [3]): - Female ICR mice were administered a single oral dose of Acadesine (150 mg/kg, 300 mg/kg, 600 mg/kg). Mice were monitored for 7 days. No mortality was observed at 150 mg/kg or 300 mg/kg; 600 mg/kg caused 20% mortality (1/5 mice). Mild toxicity signs (lethargy, reduced food intake) at 300 mg/kg resolved within 48 hours. Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels remained within the normal range [3] 2. Subchronic toxicity in rats (from [2]): - Male SD rats were administered Acadesine (50 mg/kg, 100 mg/kg, intraperitoneal injection) once daily for 28 days. No significant changes in body weight, food intake, or organ weight (liver, kidney, heart) were observed. Histological examination of major organs showed no abnormal lesions, and serum blood urea nitrogen (BUN) and creatinine (Cr) levels were normal [2] 3. Plasma protein binding (from [1]): - Plasma protein binding of Acadesine was measured via ultrafiltration (30 kDa cutoff membrane). Rat plasma was spiked with Acadesine (0.1 μM, 1 μM, 10 μM). After ultrafiltration, the concentration of Acadesine in the filtrate and plasma was measured via HPLC. The binding rate was <10% across all concentrations, indicating minimal plasma protein binding [1] |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Acadesine has been shown to induce cell death apoptosis selectively in B-cells taken from healthy subjects and patients with B-CLL, with little effect on T-cells. As T-cells have an important role in fighting infection, it is anticipated that patients treated with acadesine will have a reduced risk of serious infections compared to those on current chemotherapies. 1. Mechanism of action (from [1]): Acadesine is a nucleoside analog that is converted to ZMP (5-aminoimidazole-4-carboxamide ribonucleotide) in cells. ZMP mimics AMP to allosterically activate AMPK, which in turn phosphorylates downstream substrates (e.g., ACC) to regulate glucose and lipid metabolism. It does not directly bind to AMPK but acts via intracellular ZMP accumulation [1] 2. Research application in hematology (from [2]): Acadesine is used as a tool compound to study the role of AMPK in leukemia cell proliferation and survival. Its ability to induce G2/M arrest in HL-60 cells suggests potential for combining with other chemotherapeutic agents to enhance antitumor efficacy, though it has not advanced to clinical trials for leukemia [2] 3. Metabolic disease research value (from [3]): Due to its ability to activate AMPK and improve glucose/lipid metabolism, Acadesine is widely used in preclinical studies of type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). It serves as a positive control for AMPK activators and helps validate AMPK as a therapeutic target for metabolic disorders [3] 4. Development status (from [1][2][3]): Acadesine has not been approved for clinical use in humans. It is primarily used as a research tool to investigate AMPK-mediated signaling pathways and cellular energy metabolism, rather than a candidate drug, due to its relatively low potency and short half-life [1][2][3] |
| 分子式 |
C9H14N4O5
|
|
|---|---|---|
| 分子量 |
258.2313
|
|
| 精确质量 |
258.096
|
|
| 元素分析 |
C, 41.86; H, 5.46; N, 21.70; O, 30.98
|
|
| CAS号 |
2627-69-2
|
|
| 相关CAS号 |
AICAR phosphate;681006-28-0;AICAR-13C2,15N;1609374-70-0
|
|
| PubChem CID |
17513
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
2.1±0.1 g/cm3
|
|
| 沸点 |
726.3±60.0 °C at 760 mmHg
|
|
| 熔点 |
214-215 °C
|
|
| 闪点 |
393.1±32.9 °C
|
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
|
| 折射率 |
1.821
|
|
| LogP |
-2.93
|
|
| tPSA |
156.85
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
330
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
O1[C@]([H])(C([H])([H])O[H])[C@]([H])([C@]([H])([C@]1([H])N1C([H])=NC(C(N([H])[H])=O)=C1N([H])[H])O[H])O[H]
|
|
| InChi Key |
RTRQQBHATOEIAF-UUOKFMHZSA-N
|
|
| InChi Code |
InChI=1S/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1
|
|
| 化学名 |
5-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~51 mg/mL (~197.5 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (8.05 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (8.05 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (8.05 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2% DMSO+40% PEG 300+2% Tween 80+ddH2O: 6mg/mL 配方 5 中的溶解度: 110 mg/mL (425.98 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8725 mL | 19.3626 mL | 38.7252 mL | |
| 5 mM | 0.7745 mL | 3.8725 mL | 7.7450 mL | |
| 10 mM | 0.3873 mL | 1.9363 mL | 3.8725 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00559624 | Completed | Drug: Acadesine | Leukemia, B-Cell, Chronic | Advancell - Advanced In Vitro Cell Technologies, S.A. |
December 2007 | Phase 1 Phase 2 |
|
|
|
|