| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Heat shock protein 90 (Hsp90) (Ki = 1.7 nM for human Hsp90α; Ki = 1.3 nM for human Hsp90β) [1]
Nuclear factor kappa B (NF-κB) (indirect target) [2] Multidrug resistance-related proteins (P-gp, BCRP, MRP1) (indirect targets) [4] |
|---|---|
| 体外研究 (In Vitro) |
BIIB021 与 Hsp90 的 ATP 结合袋结合并阻碍其伴侣活性,导致客户蛋白降解并抑制肿瘤生长。 BIIB021 的 IC50 为 0.06-0.31 μM,抑制肿瘤细胞(BT474、MCF-7、N87、HT29、H1650、H1299、H69 和 H82)的增殖。当 BIIB021 存在时,热休克蛋白 Hsp70 和 Hsp27 的表达更加频繁,并且还会导致 Hsp90 客户蛋白(如 HER-2、Akt 和 Raf-1)的降解 [1]。 BIIB021 抑制霍奇金淋巴瘤细胞(KM-H2、L428、L540、L540cy、L591、L1236 和 DEV),IC50 为 0.24-0.8 μM。在健康人的淋巴细胞中,BIIB021 表现出最小的活性。尽管存在 IκB 缺陷,BIIB021 仍可降低 NF-κB 的组成活性。暴露于 BIIB021 的霍奇金淋巴瘤细胞表达更多激活 NK 细胞受体 NKG2D 的配体,使它们更容易受到 NK 细胞介导的死亡的影响 [2]。通过增加 HNSCCA 细胞系(UM11B 和 JHU12)的体外放射敏感性,BIIB021 还降低重要放射反应蛋白的表达,促进 G2 停滞并增加细胞死亡 [3]。对于肾上腺皮质癌 H295R,BIIB021 的活性远高于 17-AAG。 NQO1 缺失或 Bcl-2 过表达对 BIIB021 的细胞毒作用没有影响,分子损伤与 17-AAG 细胞死亡减少有关,但并不能阻止客户流失。此外,17-AAG 耐药细胞系(NIH-H69、MES SA Dx5、NCI-ADR-RES、Nalm6)表现出 BIIB021 的活性 [4]。
在多种人肿瘤细胞系(肺癌:A549;结直肠癌:HCT116;乳腺癌:MCF-7;前列腺癌:PC-3)中,BIIB021抑制细胞增殖,MTT法检测IC50值为0.05–0.3 μM[1] 通过泛素-蛋白酶体途径诱导Hsp90客户蛋白(EGFR、HER2、AKT、RAF-1)降解(Western blot检测),0.2 μM浓度处理24小时后EGFR蛋白水平降低>60%[1] 在霍奇金淋巴瘤细胞系(L428、HDLM2)中,BIIB021耗竭NF-κB(p65亚基)蛋白(0.1 μM处理24小时降低70%),并下调NF-κB靶基因(IL-6、TNF-α)表达(qPCR检测)[2] 药物增强淋巴瘤细胞对自然杀伤(NK)细胞介导的细胞毒性:效应细胞:靶细胞=10:1时,NK细胞杀伤率从单独NK组的25%升高至NK+0.05 μM BIIB021组的55%[2] 在头颈部鳞状细胞癌(HNSCC)细胞系(SCC-25、FaDu)中,BIIB021抑制细胞生长(MTT法IC50=0.08–0.12 μM)并增强放射敏感性(增敏比=1.4–1.6)[3] 0.1 μM BIIB021联合2 Gy放疗时,HNSCC细胞克隆形成率降低约80%,而单独放疗组仅降低约30%[3] Western blot检测显示,经BIIB021处理的放疗后HNSCC细胞中,DNA修复蛋白Ku70和抗凋亡蛋白Bcl-2表达下调[3] 在多药耐药(MDR)肿瘤细胞系(P-糖蛋白过表达KB-V1;BCRP过表达MCF-7/MX;MRP1过表达H69AR)中,BIIB021仍保持抗增殖活性,IC50值(0.06–0.35 μM)与亲本细胞(0.05–0.3 μM)相近[4] 药物下调多药耐药相关蛋白(P-糖蛋白、BCRP、MRP1)表达并逆转耐药:KB-V1细胞中多柔比星的IC50从单独用药组的180 nM降至多柔比星+0.1 μM BIIB021组的35 nM[4] BIIB021诱导MDR细胞凋亡(Annexin V/PI染色:0.2 μM处理48小时凋亡率约40%),机制涉及caspase-3激活和PARP剪切[4] |
| 体内研究 (In Vivo) |
在许多肿瘤异种移植模型中,例如 N87、BT474、CWR22、U87、SKOV3 和 Panc-1,口服 BIIB021 可抑制肿瘤生长[1]。 120 mg/kg的BIIB021剂量可有效抑制L540cy肿瘤的生长[2]。 BIIB021 显着增强了 JHU12 异种移植物中放射的抗肿瘤生长作用[3]。
在携带A549肺癌异种移植瘤的裸鼠中,口服BIIB021(50 mg/kg,每日一次,连续21天),肿瘤生长较溶媒对照组抑制约75%[1] 肿瘤组织中Hsp90客户蛋白(EGFR、AKT)水平降低,TUNEL实验显示凋亡细胞比例约30%,而溶媒对照组仅约5%[1] 在携带SCC-25 HNSCC异种移植瘤的裸鼠中,口服BIIB021(30 mg/kg,每日一次,连续14天)联合放疗(2 Gy/次,共5次),肿瘤生长抑制率约85%,单独放疗组约40%,单独BIIB021组约55%[3] 联合治疗组小鼠中位生存期较溶媒+放疗组延长20天[3] 在携带KB-V1 MDR异种移植瘤的裸鼠中,口服BIIB021(40 mg/kg,每日一次,连续18天)联合多柔比星(5 mg/kg,静脉注射,第1、8天),肿瘤体积减少约70%,单独多柔比星组减少约20%,单独BIIB021组减少约50%[4] 随访30天期间,联合治疗组未观察到明显肿瘤复发[4] |
| 酶活实验 |
将重组人Hsp90α/β与系列浓度的BIIB021及ATP(底物)在反应缓冲液中37°C孵育45分钟[1]
采用比色法检测ADP生成量以评估Hsp90 ATP酶活性,通过绘制抑制曲线计算Ki值[1] 采用表面等离子体共振(SPR)技术,将Hsp90固定在传感器芯片上,注入0.1–100 nM浓度的BIIB021,测定结合亲和力(KD值)[1] 使用1–100 μM ATP进行竞争实验,证实BIIB021结合于Hsp90的ATP结合口袋[1] |
| 细胞实验 |
多种肿瘤细胞系(A549、HCT116、MCF-7、PC-3)以3×10^3个细胞/孔接种于96孔板,以1×10^5个细胞/孔接种于6孔板[1]
用BIIB021(0.01–1 μM)或溶媒(DMSO)处理细胞,在37°C、5% CO2条件下孵育48–72小时[1] MTT法检测细胞增殖(570 nm吸光度);Western blot检测客户蛋白(EGFR、HER2、AKT)水平;Annexin V/PI染色结合流式细胞术分析凋亡[1] 霍奇金淋巴瘤细胞(L428、HDLM2)以5×10^4个细胞/孔接种于96孔板,用BIIB021(0.01–0.5 μM)处理24–48小时[2] Western blot检测NF-κB(p65)蛋白水平;qPCR定量IL-6/TNF-α mRNA水平;乳酸脱氢酶(LDH)释放法评估NK细胞介导的细胞毒性[2] HNSCC细胞(SCC-25、FaDu)以4×10^3个细胞/孔接种于96孔板,或2×10^5个细胞/孔接种于6孔板,用BIIB021(0.01–0.2 μM)处理24小时后进行放疗(0–8 Gy)[3] 放疗后培养14天,结晶紫染色进行克隆形成实验;Western blot检测Ku70/Bcl-2蛋白水平;TUNEL实验计数凋亡细胞[3] MDR细胞(KB-V1、MCF-7/MX、H69AR)以3×10^3个细胞/孔接种于96孔板,用BIIB021(0.01–0.5 μM)单独处理或与化疗药物(多柔比星、紫杉醇)联合处理[4] MTT法检测细胞活力;Western blot检测MDR相关蛋白(P-糖蛋白、BCRP、MRP1)水平;Western blot分析caspase-3/PARP剪切以证实凋亡[4] |
| 动物实验 |
Dissolved in Phospho-lipon/sucrose emulsion; 31, 62.5, and 125 mg/kg; oral gavage
N87, BT474, CWR22, U87, SKOV3 and Panc-1 tumor models in BALB/c and athymic mice Nude mice (6–7 weeks old) were subcutaneously injected with 2×10^6 A549 lung cancer cells to establish xenografts [1] When tumors reached 100–150 mm³, mice were randomized into vehicle (n=8) and BIIB021 treatment groups (n=8) [1] BIIB021 was dissolved in 0.5% methylcellulose + 0.2% Tween 80 and administered orally at 50 mg/kg, once daily for 21 days [1] Vehicle group received equal volumes of 0.5% methylcellulose + 0.2% Tween 80 [1] Tumor volume was measured every 2 days; mice were euthanized at study end, and tumors were harvested for Western blot (client proteins) and TUNEL assay [1] Nude mice were subcutaneously injected with 3×10^6 SCC-25 HNSCC cells to establish xenografts [3] Tumor-bearing mice were divided into 4 groups (n=6/group): vehicle, BIIB021 alone, radiation alone, BIIB021 + radiation [3] BIIB021 (30 mg/kg, oral, once daily) was given for 14 days; radiation (2 Gy/fraction, 5 fractions) was delivered on days 1–5 [3] Tumor volume and mouse survival were monitored; tumor tissues were collected for histopathological analysis and Western blot (Ku70/Bcl-2) [3] Nude mice were subcutaneously injected with 2×10^6 KB-V1 MDR cells to establish xenografts [4] Mice were assigned to 4 groups (n=7/group): vehicle, BIIB021 alone (40 mg/kg, oral, once daily for 18 days), doxorubicin alone (5 mg/kg, iv, days 1 and 8), BIIB021 + doxorubicin [4] Tumor volume was measured every 3 days; recurrence was monitored for 30 days post-treatment; tumors were harvested for Western blot (P-gp) and TUNEL assay [4] |
| 药代性质 (ADME/PK) |
BIIB021 is orally bioavailable with a bioavailability of ~45% in mice [1]
The plasma elimination half-life (t1/2) is ~6.2 hours in mice after oral administration (50 mg/kg) [1] It is primarily metabolized by hepatic cytochrome P450 enzymes (CYP3A4 and CYP2C9), with minor contributions from CYP2D6 [1] The drug distributes widely to tissues, with tumor/plasma concentration ratio of ~4:1 at 4 hours post-oral dosing [1] Renal excretion accounts for ~15% of total elimination, with the majority of drug eliminated as metabolites in feces [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In xenograft mice treated with BIIB021 (30–50 mg/kg oral, daily for up to 21 days), no significant body weight loss (>10%) or mortality was observed [1][3][4]
Histopathological examination of liver, kidney, heart, lung, and spleen showed no overt toxic lesions or inflammation [1][3] Plasma protein binding rate is ~97% (measured in human plasma via equilibrium dialysis) [1] Co-administration with CYP3A4 inhibitors (e.g., ketoconazole) increased BIIB021 plasma exposure by ~2.3-fold in mice, indicating potential drug-drug interactions [1] |
| 参考文献 |
|
| 其他信息 |
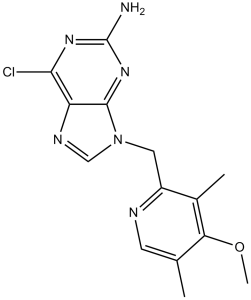
BIIB021 is a member of the class of 2-aminopurines that is 2-aminopurine which is substituted by a chlorine at position 6 and by a (4-methoxy-3,5-dimethylpyridin-2-yl)methyl group at position 9. It has a role as a Hsp90 inhibitor and an antineoplastic agent. It is a member of pyridines, a member of 2-aminopurines, an organochlorine compound and an aromatic ether.
BIIB021 has been investigated for the treatment of Tumors and Lymphoma. Hsp90 Inhibitor BIIB021 is an orally active inhibitor of heat shock protein 90 (HSP90) with potential antineoplastic activity. HSP90, a chaperon protein upregulated in a variety of tumor cells, regulates the folding and degradation of many oncogenic signaling proteins. HSP90 inhibitor BIIB021 specifically blocks active HSP90, thereby inhibiting its chaperon function and promoting the degradation of oncogenic signaling proteins involved in tumor cell proliferation and survival. As a result, CNF2024 has the potential to inhibit the growth of a wide range of cancer cells in both solid tumors and blood-based cancers. BIIB021 is an orally available, fully synthetic small-molecule inhibitor of Hsp90 [1] Its mechanism of action involves binding to the ATP-binding pocket of Hsp90, disrupting chaperone function and promoting proteasomal/lysosomal degradation of oncogenic client proteins [1][2][3][4] It exhibits broad anti-tumor activity against solid tumors (lung, colon, breast, prostate, HNSCC) and hematologic malignancies (Hodgkin's lymphoma) [1][2][3] The drug overcomes multidrug resistance by downregulating MDR-related transporters (P-gp, BCRP, MRP1) and restoring sensitivity to chemotherapeutics [4] It sensitizes HNSCC cells to radiotherapy by inhibiting DNA repair and downregulating anti-apoptotic proteins [3] In Hodgkin's lymphoma, it enhances NK cell-mediated cytotoxicity via NF-κB depletion, which reduces tumor cell expression of immune checkpoint molecules [2] |
| 分子式 |
C
|
|---|---|
| 分子量 |
318.76
|
| 精确质量 |
318.099
|
| CAS号 |
848695-25-0
|
| 相关CAS号 |
1225041-97-3 (mesylate);848695-25-0;
|
| PubChem CID |
16736529
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
588.5±60.0 °C at 760 mmHg
|
| 熔点 |
192-193℃
|
| 闪点 |
309.7±32.9 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.711
|
| LogP |
1.66
|
| tPSA |
91.74
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
388
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
QULDDKSCVCJTPV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H15ClN6O/c1-7-4-17-9(8(2)11(7)22-3)5-21-6-18-10-12(15)19-14(16)20-13(10)21/h4,6H,5H2,1-3H3,(H2,16,19,20)
|
| 化学名 |
[6-Chloro-9-(4-methoxy-3,5-dimethylpyridin-2-ylmethyl)-9H-purin-2-yl]amine
|
| 别名 |
CNF2024; BIIB-021; CNF-2024; CNF 2024; BIIB021; BIIB 021;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.84 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.84 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1372 mL | 15.6858 mL | 31.3716 mL | |
| 5 mM | 0.6274 mL | 3.1372 mL | 6.2743 mL | |
| 10 mM | 0.3137 mL | 1.5686 mL | 3.1372 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00618735 | Completed | Drug: BIIB021 | Advanced Solid Tumors | Biogen | February 2008 | Phase 1 |
| NCT00618319 | Completed | Drug: BIIB021 | GIST | Biogen | February 2008 | Phase 2 |
| NCT00344786 | Terminated | Drug: CNF2024 (BIIB021) | B-Cell Chronic Lymphocytic Leukemia | Biogen | February 2006 | Phase 1 |
| NCT00412412 | Completed | Drug: CNF2024 Drug: CNF2024 + trastuzumab |
Breast Cancer | Biogen | December 2007 | Phase 1 |
|
|---|
|
|