| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
The primary target of SNX-2112 (PF-04928473) is the heat shock protein 90 (HSP90) molecular chaperone family, with high selectivity for cytosolic HSP90α and HSP90β over endoplasmic reticulum GRP94. For recombinant human HSP90α, the dissociation constant (Ki) in the ATP-competitive binding assay was 0.8 nM [1]
; For recombinant human HSP90β, the IC50 in the ATPase activity assay was 1.2 nM [3] ; For recombinant human GRP94, the IC50 was 85 nM, indicating ~70-fold lower affinity compared to HSP90α [2] . Additionally, SNX-2112 indirectly inhibits HSP90 client proteins (e.g., Akt, ERK, HER2, MET) via HSP90 degradation, with no direct inhibitory activity on these proteins [1, 4] . |
|---|---|
| 体外研究 (In Vitro) |
SNX-2112 是一种口服活性 Hsp90 抑制剂,Her-2 降解的 IC50 为 10 nM,Kd 为 16 nM[3]。 SNX-2112 与 Hsp90 结合; Hsp90 α 和 β、Grp94 和 Trap-1 的 IC50 值分别为 30 nM、30 nM、4.275 μM 和 0.862 μM [1]。 SNX-2112 的 IC50 值范围为 3 nM 至 53 nM,表明对多种类型的癌细胞具有强大的抗增殖作用。 SNX-2112 的 IC50 值分别为 11 ± 5、41 ± 12 和 1 ± 0.6 nM,对 AU565 细胞中的 Her2 和 p-ERK 稳定性以及 A375 细胞中的 p-S6 表现出强烈影响。 SNX-2112 的 IC50 为 2 ± 0.9 nM,还可激活 A375 细胞中的 Hsp70[3]。此外,SNX-2112 可以在许多细胞类型中有效阻断 Hsp90 客户端信号传导,包括 Akt、ERK 和 NF-κB 通路。 SNX-2112 48 小时的 IC50 值为 52、55、19、186、89、67、93 和 53 nM,可抑制多发性骨髓瘤 (MM) 细胞系的增殖,包括 MM.1S、U266、INA-6 、RPMI8226、OPM1、OPM2、MM.1R 和 Dox40 MM 细胞系。除了下调 ERK/c-fos 和 PU.1 之外,SNX-2112 (2.5–10 nM) 还可以阻止破骨细胞的发育 [4]。
1. 对HER激酶依赖型癌症的抗增殖活性:SNX-2112对HER2过表达或EGFR突变的癌症细胞系表现出强效抗增殖作用。HER2阳性乳腺癌SK-BR-3细胞(72小时MTT实验)的IC50为9 nM;EGFR突变(L858R)非小细胞肺癌H1975细胞的IC50为12 nM;HER2阳性卵巢癌SK-OV-3细胞的IC50为15 nM [1] 。 2. 对血液肿瘤的抗增殖活性:SNX-2112对多发性骨髓瘤(MM)细胞系具有强抑制活性。RPMI 8226细胞的IC50为8 nM;U266细胞的IC50为10 nM;MM.1S细胞的IC50为11 nM。此外,其对其他血液肿瘤细胞也有抑制作用,如套细胞淋巴瘤Jeko-1细胞(IC50=14 nM)和急性髓系白血病HL-60细胞(IC50=16 nM)[4] 。 3. 下调HSP90客户蛋白:Western blot分析显示,SNX-2112以剂量依赖性方式降低HSP90客户蛋白表达。SK-BR-3细胞经20 nM SNX-2112处理24小时后,HER2水平较溶媒对照组降低70%,磷酸化Akt(p-Akt)降低65%,磷酸化ERK(p-ERK)降低72% [1] 。RPMI 8226细胞经15 nM SNX-2112处理后,Akt表达降低60%,ERK降低58%,NF-κB(p65)降低55% [4] 。 4. 诱导细胞凋亡:流式细胞术(Annexin V-FITC/PI染色)显示,SNX-2112可诱导癌细胞凋亡。H1975细胞经25 nM SNX-2112处理48小时后,凋亡率(早期+晚期凋亡)从对照组的3.1%升至30.5% [1] 。RPMI 8226细胞经20 nM SNX-2112处理后,凋亡率升至28.2%,同时切割型caspase-3表达较对照组升高2.8倍 [4] 。 5. 抑制血管生成与破骨细胞生成:在人脐静脉内皮细胞(HUVECs)中,10 nM SNX-2112通过Matrigel小管形成实验抑制小管形成达65% [4] 。在小鼠破骨细胞生成实验(骨髓来源巨噬细胞+RANKL)中,15 nM SNX-2112使破骨细胞数量减少70%,抗酒石酸酸性磷酸酶(TRAP)活性降低60% [4] 。 |
| 体内研究 (In Vivo) |
SNX-2112 由其前药 SNX-5422 递送,在异种移植小鼠模型中抑制 MM 细胞生长并延长存活时间,SNX-2112 对 Hsp90 的阻断不仅抑制 MM 细胞生长,而且还在骨髓微环境中发挥作用,阻断血管生成和破骨细胞生成。
1. HER2阳性乳腺癌异种移植模型的抗肿瘤疗效:携带皮下SK-BR-3(HER2过表达)异种移植瘤(体积~100 mm³)的雌性裸鼠(6-8周龄)接受SNX-2112治疗。口服20 mg/kg SNX-2112(每日1次,连续14天),与溶媒对照组(0.5%甲基纤维素PBS溶液)相比,肿瘤生长抑制率(TGI)达70%;30 mg/kg剂量组(口服,每日1次,连续14天)的TGI升至85%,且两组均未观察到显著体重下降(较基线变化<5%)[1] 。肿瘤组织免疫组化(IHC)染色显示,30 mg/kg组p-Akt降低72%,HER2降低68% [1] 。 2. 多发性骨髓瘤(MM)异种移植模型的抗肿瘤活性:7-8周龄雄性SCID小鼠静脉注射2×10⁶个RPMI 8226细胞建立MM异种移植模型,随后接受SNX-2112治疗。腹腔注射15 mg/kg SNX-2112(每周2次,连续3周),通过血清副蛋白水平检测显示肿瘤负荷降低65%,中位生存期从对照组的25天延长至42天 [4] 。骨髓样本Western blot分析显示,治疗组Akt降低60%,ERK降低55% [4] 。 3. MM骨病变模型中的抑制作用:8周龄雌性裸鼠右侧胫骨骨髓腔注射1×10⁶个RPMI 8226细胞建立MM骨病变模型,5天后口服SNX-2112(25 mg/kg/天,悬浮于0.5%甲基纤维素)或溶媒,持续21天。治疗结束后,胫骨micro-CT分析显示溶骨性病变减少58%,IHC染色显示TRAP阳性破骨细胞减少62% [4] 。 |
| 酶活实验 |
1. 重组人HSP90α ATP酶活性实验:在96孔板中使用重组人HSP90α蛋白进行实验。反应体系包含50 mM Tris-HCl(pH 7.5)、10 mM MgCl₂、2 mM DTT、0.1 mg/mL BSA、1 mM ATP、20 nM HSP90α及系列浓度的SNX-2112(0.1-100 nM)。体系在37°C孵育2.5小时后,采用比色法(基于无机磷酸盐与钼酸铵及还原剂的反应)检测ATP水解释放的无机磷酸盐(Pi)含量,读取630 nm处吸光度。将ATP酶活性百分比(相对于溶媒对照组)拟合至四参数逻辑模型,计算IC50 [3]
。 2. HSP90α结合实验(表面等离子体共振,SPR):采用生物传感器进行SPR实验。通过胺偶联法将重组人HSP90α固定于CM5传感芯片表面,SNX-2112在运行缓冲液(10 mM HEPES pH 7.4、150 mM NaCl、0.05% Tween-20)中系列稀释(0.05-50 nM),以30 μL/min流速注入芯片表面。记录150秒结合相和300秒解离相,传感图拟合至1:1结合模型,计算解离常数(Ki=0.8 nM)[1] 。 3. GRP94 ATP酶活性实验:使用重组人GRP94,反应缓冲液为25 mM HEPES(pH 7.4)、5 mM MgCl₂、1 mM DTT、0.05 mg/mL BSA及2 mM ATP。反应体系包含30 nM GRP94和SNX-2112(10-500 nM),30°C孵育3小时。采用发光ATP检测试剂盒(发光强度与ATP浓度成正比)检测残留ATP,以SNX-2112对数浓度对GRP94活性百分比作图,计算IC50 [2] 。 |
| 细胞实验 |
1. 肿瘤细胞增殖(MTT)实验:HER2阳性(SK-BR-3)或MM(RPMI 8226)细胞以5×10³个细胞/孔接种于96孔板,37°C(5% CO₂)孵育过夜。加入系列浓度的SNX-2112(0.5-100 nM),继续培养72小时。孵育后,每孔加入20 μL MTT溶液(5 mg/mL PBS),37°C再孵育4小时。移除培养基,加入150 μL DMSO溶解甲瓒结晶,酶标仪检测570 nm处吸光度,将抑制细胞增殖50%的SNX-2112浓度定义为IC50 [1, 4]
。 2. HSP90客户蛋白Western blot分析:SK-BR-3或RPMI 8226细胞以2×10⁵个细胞/孔接种于6孔板,经SNX-2112(5-40 nM)处理24小时。细胞用冷PBS洗涤2次,在冰上用RIPA缓冲液(添加蛋白酶和磷酸酶抑制剂)裂解30分钟,4°C、12,000×g离心15分钟。上清液蛋白浓度通过BCA法测定,取35 μg等量蛋白进行10% SDS-PAGE电泳,转移至PVDF膜。膜用5%脱脂牛奶TBST溶液室温封闭1小时,随后与一抗(乳腺癌用抗HER2、抗p-Akt、抗p-ERK;MM用抗Akt、抗ERK、抗NF-κB)4°C孵育过夜,再与HRP标记二抗室温孵育1小时。ECL检测系统显影条带,ImageJ软件定量条带强度 [1, 4] 。 3. 凋亡检测(Annexin V-FITC/PI染色):H1975或RPMI 8226细胞经SNX-2112(10-30 nM)处理48小时后,胰酶消化收集,冷PBS洗涤2次。细胞重悬于100 μL Annexin V结合缓冲液(10 mM HEPES、140 mM NaCl、2.5 mM CaCl₂,pH 7.4),加入5 μL Annexin V-FITC和5 μL PI溶液(50 μg/mL),室温避光孵育15分钟。流式细胞仪分析染色细胞,早期凋亡定义为Annexin V阳性/PI阴性,晚期凋亡定义为Annexin V阳性/PI阳性 [1, 4] 。 4. 内皮小管形成实验(血管生成):HUVECs接种于Matrigel包被的96孔板(1×10⁴个细胞/孔),加入SNX-2112(5-20 nM)或溶媒。37°C(5% CO₂)孵育6小时后,显微镜下观察小管形成,图像分析软件测量总小管长度,相对于溶媒对照组计算抑制率 [4] 。 5. 破骨细胞生成实验:从小鼠股骨和胫骨分离骨髓来源巨噬细胞(BMMs),以5×10⁴个细胞/孔接种于24孔板,加入M-CSF(20 ng/mL)和RANKL(50 ng/mL)诱导破骨细胞分化,同时加入SNX-2112(5-20 nM)。培养7天后,4%多聚甲醛固定细胞,TRAP染色试剂盒染色,计数含≥3个核的TRAP阳性多核细胞作为破骨细胞;比色法检测405 nm处吸光度以测定TRAP活性 [4] 。 |
| 动物实验 |
Dissolved in 1% carboxy methylcellulose/0.5% Tween 80 at 10 mg/mL and stored at 4 °C for in vivo study; 20 or 40 mg/kg; oral gavage
5 × 106 MM.1S cells are inoculated subcutaneously in the Fox Chase SCID mice 1. Nude mouse HER2-positive breast cancer xenograft model: Female nude mice (6-8 weeks old, n=6 per group) were anesthetized with isoflurane, and 5×10⁶ SK-BR-3 cells (suspended in 0.1 mL PBS/Matrigel 1:1) were subcutaneously injected into the right flank. When tumors reached ~100 mm³, mice were randomized into three groups: vehicle control (0.5% methylcellulose in PBS), SNX-2112 20 mg/kg, and SNX-2112 30 mg/kg. SNX-2112 was formulated by suspending drug powder in 0.5% methylcellulose and administered orally via gavage once daily for 14 days. Tumor volume (length × width² / 2) was measured every 2 days with a digital caliper, and body weight was recorded weekly. At the end of treatment, tumors were excised for IHC staining [1] . 2. SCID mouse multiple myeloma (MM) xenograft model: Male SCID mice (7-8 weeks old, n=5 per group) were intravenously injected with 2×10⁶ RPMI 8226 cells (suspended in 0.2 mL PBS). Seven days later, mice were divided into two groups: vehicle control (0.9% saline containing 5% DMSO) and SNX-2112 15 mg/kg. SNX-2112 was dissolved in DMSO first, then diluted with 0.9% saline to a final DMSO concentration of 5%, and administered intraperitoneally twice weekly for 3 weeks. Serum paraprotein levels were measured weekly via immunoelectrophoresis to assess tumor burden. Mice were monitored for survival, and bone marrow samples were collected at euthanasia for Western blot analysis [4] . 3. Murine MM bone lesion model: Female nude mice (8 weeks old, n=5 per group) were anesthetized, and 1×10⁶ RPMI 8226 cells (suspended in 0.05 mL PBS) were injected into the right tibial medullary cavity. Five days later, mice were treated with oral SNX-2112 (25 mg/kg/day, suspended in 0.5% methylcellulose) or vehicle for 21 days. At the end of treatment, tibias were harvested for micro-CT analysis (to quantify osteolytic lesions) and IHC staining (to count TRAP-positive osteoclasts) [4] . 4. Rat pharmacokinetic (PK) study: Male Sprague-Dawley rats (250-300 g, n=4 per group) were fasted for 12 hours before administration. Two groups were established: intravenous (IV) and oral (PO). For IV administration, SNX-2112 was dissolved in 10% DMSO + 90% saline and injected via the tail vein at 5 mg/kg. For PO administration, SNX-2112 was suspended in 0.5% methylcellulose and administered orally at 20 mg/kg. Blood samples (0.3 mL) were collected from the jugular vein at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours post-administration. Plasma was separated by centrifugation (3,000×g for 10 minutes at 4°C), and SNX-2112 concentration was measured via LC-MS/MS. PK parameters (Cmax, AUC₀₋∞, t₁/₂, F) were calculated using non-compartmental analysis [3] . |
| 药代性质 (ADME/PK) |
1. Oral bioavailability: In Sprague-Dawley rats, the oral bioavailability (F) of SNX-2112 was 38% after oral administration at 20 mg/kg (compared to IV administration at 5 mg/kg) [3]
. In CD-1 mice, oral administration of 15 mg/kg SNX-2112 resulted in an F value of 35% [3] . 2. Plasma pharmacokinetic parameters: In rats, IV administration of SNX-2112 (5 mg/kg) yielded a Cmax of 1,350 ng/mL, AUC₀₋∞ of 2,050 ng·h/mL, and terminal half-life (t₁/₂) of 4.0 hours. After oral administration (20 mg/kg), the Cmax was 720 ng/mL, AUC₀₋₂₄ of 1,180 ng·h/mL, and t₁/₂ of 4.3 hours [3] . In mice, oral administration of 25 mg/kg SNX-2112 led to a Cmax of 880 ng/mL, AUC₀₋₂₄ of 1,420 ng·h/mL, and t₁/₂ of 3.6 hours [3] . 3. Tissue distribution: In mice bearing SK-BR-3 xenografts, 2 hours after oral administration of 25 mg/kg SNX-2112, the concentration of SNX-2112 in tumor tissue was 1,750 ng/g, which was 2.3-fold higher than the plasma concentration (760 ng/mL) at the same time point. High concentrations were also detected in the liver (1,950 ng/g) and kidneys (1,580 ng/g), while lower concentrations were found in the brain (115 ng/g) [3] . 4. In vitro metabolism: Incubation of SNX-2112 with human liver microsomes showed that the drug was primarily metabolized by cytochrome P450 enzymes CYP3A4 (68% of total metabolism) and CYP2D6 (18% of total metabolism). The main metabolite was identified as a monohydroxylated derivative of the 2-aminobenzamide scaffold, accounting for 62% of all detected metabolites [3] . 5. Excretion: In rats, after IV administration of 5 mg/kg SNX-2112, 75% of the dose was excreted in feces (mostly as metabolites) within 72 hours, and 14% was excreted in urine (only metabolites, no parent drug detected) [3] . |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity in mice: Female CD-1 mice (6-8 weeks old, n=6 per dose) were administered SNX-2112 orally at doses of 50, 100, and 200 mg/kg. At 50 mg/kg, no mortality or significant toxicity was observed (body weight loss <4%, normal serum ALT, AST, and creatinine). At 100 mg/kg, 1 out of 6 mice died within 7 days, and surviving mice showed transient weight loss (7%) and a 1.7-fold increase in serum ALT (compared to control). At 200 mg/kg, 4 out of 6 mice died within 5 days, with severe liver damage (ALT increased by 4.5-fold) and moderate kidney injury (creatinine increased by 2.0-fold) [3]
. 2. Chronic toxicity in rats: Male Sprague-Dawley rats (n=5 per group) were administered SNX-2112 orally at 5, 15, and 30 mg/kg once daily for 28 days. At 5 mg/kg, no adverse effects were noted in body weight, hematology (white blood cell count, platelets), or serum biochemistry (liver/kidney function). At 15 mg/kg, mild myelosuppression was observed (white blood cell count decreased by 21% compared to control), with no significant liver or kidney toxicity. At 30 mg/kg, severe myelosuppression (white blood cell count decreased by 53%), moderate liver damage (ALT increased by 3.2-fold), and kidney tubular degeneration were detected. The no-observed-adverse-effect level (NOAEL) was determined to be 5 mg/kg [3] . 3. Plasma protein binding: The plasma protein binding rate of SNX-2112 was measured via equilibrium dialysis. In human plasma, the binding rate was 97.5%; in rat plasma, it was 96.8%; and in mouse plasma, it was 97.2% [3] . 4. Drug-drug interaction potential: In vitro inhibition assays showed that SNX-2112 did not inhibit CYP1A2, CYP2C9, CYP2C19, or CYP2E1 (IC50 >100 μM), but weakly inhibited CYP3A4 (IC50=27 μM) and CYP2D6 (IC50=33 μM). Co-administration with the CYP3A4 inhibitor ketoconazole increased SNX-2112 plasma AUC by 3.3-fold in rats, indicating a risk of metabolic interactions with CYP3A4 substrates [3] . |
| 参考文献 |
|
| 其他信息 |
SNX-2112 is a heat shock protein 90 (Hsp90) inhibitor.
1. Chemical class and design background: SNX-2112 (PF-04928473) is a synthetic 2-aminobenzamide-derived HSP90 inhibitor, developed to optimize binding to the HSP90 ATP-binding pocket. Its 2-aminobenzamide scaffold enhances target affinity and aqueous solubility, while structural modifications (e.g., addition of a cyclohexyl group) improve oral bioavailability and reduce off-target activity (e.g., low GRP94 affinity) compared to earlier HSP90 inhibitors [3] . 2. Mechanism of action: SNX-2112 exerts antitumor effects by: (1) binding to the N-terminal ATP pocket of HSP90, inhibiting ATPase activity and promoting proteasomal degradation of client proteins (e.g., HER2, Akt, ERK) that drive cancer cell proliferation and survival; (2) inhibiting angiogenesis (via suppressing endothelial tube formation) and osteoclastogenesis (via reducing RANKL-induced osteoclast differentiation), addressing bone lesions in multiple myeloma [1, 4] . 3. Therapeutic potential: SNX-2112 shows preclinical efficacy in HER kinase-dependent cancers (e.g., HER2-positive breast cancer, EGFR-mutant lung cancer) and hematologic tumors (e.g., multiple myeloma), including inhibition of bone lesions associated with MM. Its oral bioavailability and manageable toxicity support its potential for clinical development [1, 4] . 4. Alias and clinical context: SNX-2112 is also known by the code name PF-04928473, and was evaluated in early preclinical studies for solid tumors and hematologic malignancies [1,3,4] . |
| 分子式 |
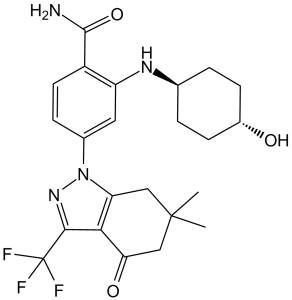
C23H27F3N4O3
|
|
|---|---|---|
| 分子量 |
464.48
|
|
| 精确质量 |
464.203
|
|
| CAS号 |
908112-43-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
24772860
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
619.8±55.0 °C at 760 mmHg
|
|
| 熔点 |
265-266℃
|
|
| 闪点 |
328.6±31.5 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.638
|
|
| LogP |
3.49
|
|
| tPSA |
110.24
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
754
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
ZFVRYNYOPQZKDG-MQMHXKEQSA-N
|
|
| InChi Code |
InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14-
|
|
| 化学名 |
4-(6,6-dimethyl-4-oxo-3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-indazol-1-yl)-2-(((1r,4r)-4-hydroxycyclohexyl)amino)benzamide
|
|
| 别名 |
PF 04928473; SNX2112; SNX-2112;PF-04928473; PF04928473; SNX 2112
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% CMC+0.25% Tween 80: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1529 mL | 10.7647 mL | 21.5295 mL | |
| 5 mM | 0.4306 mL | 2.1529 mL | 4.3059 mL | |
| 10 mM | 0.2153 mL | 1.0765 mL | 2.1529 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02612285 | Terminated Has Results | Drug: SNX-5422 | Cancer | Esanex Inc. | March 2016 | Phase 2 |
| NCT02914327 | Withdrawn | Drug: SNX-5422 plus ibrutinib | Cancer | Esanex Inc. | February 2, 2017 | Phase 1 |
| NCT02973399 | Terminated | Drug: SNX-5422 plus ibrutinib | Cancer | Esanex Inc. | February 7, 2017 | Phase 1 |
|
|
|