| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Bovine brain PKC (IC50 = 10 nM); PKCβII (IC50 = 16 nM); PKCβI (IC50 = 17 nM); PKCα(IC50 = 20 nM); PKCγ(IC50 = 20nM); FDGFR (IC50 = 65 μM)
|
|---|---|
| 体外研究 (In Vitro) |
双吲哚马来酰亚胺 I 盐酸盐 (5 μM) 可抑制 α-磷酸酶产生的 P47 磷酸化 [1]。 μM)可使脂肪细胞内质中的GSK-3活性降低至25.1±4.3%[3]。双吲哚马来酰亚胺 I 盐酸盐(10 μM,24 小时)可抑制 PC3 细胞的外泌体和微泡 (EMV) 释放 [4]。双吲哚基马来酰亚胺 I 盐酸盐(10 μM,24 小时)可增强氟尿嘧啶的 5-细胞毒性 [4]。
Staurosporine是文献中描述的最有效的蛋白激酶C (PKC)抑制剂,其半最大抑制浓度(IC50)为10 nM。然而,这种天然产物在检测其他蛋白激酶时选择性较差。为了获得特异性的PKC抑制剂,合成了一系列双吲哚基马来酰亚胺。结构-活性关系研究允许确定负责赋予高效力和缺乏选择性的硫孢素分子的亚结构。几种氨基烷基双吲哚酰马来酰亚胺被发现是有效的和选择性的PKC抑制剂(IC50值从5到70 nM)。其中的GF 109203X的进一步研究旨在表征该化学家族。GF 109203X是一种竞争性ATP抑制剂(Ki = 14 +/- 3 NM),与五种不同的蛋白激酶相比,对PKC表现出高选择性。我们进一步确定了GF 109203X在两种细胞模型中的效力和特异性:人血小板和Swiss 3T3成纤维细胞。GF 109203X有效地阻止pkc介导的血小板Mr = 47000蛋白和瑞士3T3细胞Mr = 80000蛋白的磷酸化。相反,在相同的模型中,PKC抑制剂未能阻止PKC非依赖性磷酸化。GF 109203X抑制胶原和α -凝血酶诱导的血小板聚集以及胶原引发的ATP分泌。然而,adp依赖性可逆聚集未被修饰。在Swiss 3T3成纤维细胞中,GF 109203X逆转了由phorbol 12,13-二丁酸盐诱导的表皮生长因子结合的抑制,并阻止[3H]胸苷结合到DNA中,只有当激活PKC的生长促进剂才会引起这种抑制。我们的研究结果说明了GF 109203X作为研究PKC参与信号转导途径的工具的潜力。[1] 研究人员报道了广泛使用的蛋白激酶C抑制剂,双吲哚酰马来酰亚胺I ( GF 109203X)和IX,是糖原合成酶激酶3 (GSK-3)的有效抑制剂。双吲哚酰马来酰亚胺I和IX在体外抑制GSK-3,分别在大鼠附睾脂肪细胞的细胞裂解物(IC(50) 360 nM和6.8 nM)或GSK-3 β免疫沉淀物(IC(50) 170 nM和2.8 nM)中检测。用双吲哚酰马来酰亚胺I(5微米)和IX(2微米)预处理脂肪细胞,使总细胞裂解物中的GSK-3活性分别降低至对照组的25.1+/-4.3%和12.9+/-3.0%。相比之下,双吲哚马来酰亚胺V(5微米)缺乏双吲哚马来酰亚胺I和IX上存在的官能团,效果不明显。我们提出双吲哚酰马来酰亚胺I和IX可以直接抑制GSK-3,这可能解释了一些先前报道的胰岛素样对糖原合成酶活性的影响。[3] |
| 体内研究 (In Vivo) |
在机械通气 (MV) 组的小鼠中,腹腔注射双吲哚马来酰亚胺 I 盐酸盐 (0.02 mg/kg) 可降低 NLRP3、P-PKCɑ 和 PKCɑ 升高的水平 [5]。 双吲哚马来酰亚胺 I(0-20 mg/kg,腹腔注射)可降低喹吡罗引起的鼩鼱呕吐的平均频率 [6]。
链脲佐菌素(STZ)诱导的慢性高血糖对神经血管偶联有不利影响,与PKC介导的磷酸化增加和PKC异构体表达改变有关。在这里,我们试图确定:1)选择性PKC-α/β/γ抑制剂,GF 109203X,是否可以逆转慢性高血糖对脑血管反应性的影响;2)胰岛移植可预防1型糖尿病大鼠脑血管损伤的发生。我们研究了GF109203X对糖尿病(DM)、非糖尿病(ND)和移植(TR) Lewis大鼠坐骨神经刺激(SNS)或局部应用大电导Ca2+操作的K+(BKCa)通道开启器NS1619或K+向内整流(Kir)通道激动剂KCl的影响。使用封闭颅窗在体显微镜技术监测枕小动脉直径变化。与ND或TR大鼠相比,与SNS相关的动脉小动脉扩张反应降低了约45%。此外,局部KCl和NS1619对DM大鼠的动脉动脉扩张有很大的减弱,而ND和TR动物则没有。在脑表面急性应用GF109203X后,这些反应完全恢复。PKC抑制剂对正常血糖和TR动物的血管反应没有影响。总之,dm相关的神经血管耦合慢性损伤可能很容易通过PKC-α/β/γ抑制剂逆转或通过胰岛移植预防。我们认为特定的PCK亚型(α/β/γ)与高血糖所见的神经血管解耦有机制联系。[2] <人力资源> 为检测PKC抑制剂GF 109203X/双吲哚酰马来酰亚胺I对PKC和NLRP3的影响,将雄性C57BL/6小鼠(7周龄,19 ~ 23 g)随机分为4组:对照组(C)、双吲哚酰马来酰亚胺I预处理组(B)、MV组和双吲哚酰马来酰亚胺I预处理+ MV组(B + MV)。小鼠于注射前1 h腹腔注射双吲哚酰马来酰亚胺I (0.02 mg/kg)。MV在高潮气量(30 ml/kg)下进行。为了探讨EX对VILI的改善作用,将小鼠随机分为C组、MV组、EX组和EX + MV组,分别进行MV和5周的EX训练。通气后采用苏木精-伊红(HE)染色及湿/干重比评价肺病理生理变化。Western blotting检测肺组织中PKC、P-PKC、ASC、procaspase-1、caspase-1、pro-IL-1β、IL-1β、NLRP3、occludin(紧密连接蛋白)的表达。ELISA法测定肺泡灌洗液中IL-6水平。 结果:MV组NLRP3、P-PKC α和PKC α水平升高,但GF 109203X/双吲哚酰亚胺I治疗逆转了这一变化。抑制PKC的产生可阻止NLRP3的激活。此外,MV升高ASC、procaspase-1、caspase-1、pro-IL-1β和il -1β水平,occludin水平降低,但EX减轻了这些变化。HE染色及肺损伤评分证实C组和EX组均未见明显肺损伤。MV组肺损伤最为严重,EX + MV组肺损伤有所改善。总的来说,这些发现表明,MV通过激活PKC和诱导occludin降解来激活NLRP3炎性体,而运动则减弱NLRP3炎性体和PKC的激活。此外,运动可以改善循环拉伸引起的闭塞蛋白降解。 结论:PKC激活可使NLRP3水平升高,导致肺损伤。运动可以通过抑制PKC和NLRP3的激活来减轻肺损伤。运动可能是临床预防VILI的一种潜在措施。[5] 多巴胺有5种受体亚型(D1-5),与许多神经系统疾病有关。多巴胺D2受体激动剂治疗引起恶心和呕吐。多巴胺D2受体引发呕吐的信号机制尚不清楚。在呕吐能力强的动物中,磷脂酰肌醇3-激酶(PI3K)-和蛋白激酶C (PKC)相关的信号级联刺激注射各种催吐因子后的呕吐。本研究利用最小鼩鼱研究了多巴胺D2受体介导的呕吐的潜在机制。我们发现,选择性多巴胺D2受体激动剂喹匹罗(2mg /kg,口服)引起的呕吐可被以下药物显著抑制:1)多巴胺D2偏好拮抗剂舒必利(s.c);ii)选择性PI3K抑制剂LY294002 (i.p.);iii) PKCαβII抑制剂,GF 109203X (p.);iv)细胞外信号调节蛋白激酶1/2 (ERK1/2)的选择性抑制剂,U0126 (i.p.)。喹匹罗诱发的孤束核(NTS) c-fos免疫荧光被磺胺吡啶(8mg /kg, s.c)预处理抑制。对鼩鼱脑干呕吐位点蛋白裂解物的Western blot分析显示,暴露于喹匹罗(2mg /kg, i.p) 30分钟后,Akt(蛋白激酶B (PKB))的Ser473位点磷酸化显著且时间依赖性增加。使用有效止吐剂量的舒匹利、LY294002、 GF 109203X或U0126进行预处理,可显著降低喹匹罗刺激的呕吐相关蛋白磷酸化,包括p-85PI3K、mTOR (Ser2448/2481)、PKCαβII (Thr638/641)、ERK1/2 (Thr202/204)和Akt (Ser473)。我们的研究结果证实了PI3K/mTOR/Akt和PI3K/PKCαβII/ERK1/2/Akt信号通路在多巴胺D2受体介导的呕吐中的作用。潜在的新型止吐药靶向与这些信号级联相关的呕吐蛋白,可能提供增强的效力和/或对呕吐的疗效。[6] |
| 酶活实验 |
PKC的测定通过测量从[γ-32Pi]ATP转移到富含赖氨酸的组蛋白Ill-s型的32Pi来排列。反应混合物(80μL)含有50mM Tris-HCI,pH 7.4。100μM CaCl2、10 mM MgCI2、37.5μg/mL组蛋白型Ill-s、10μM[γ-32Pi]ATP(1250cpm/pmol)、31μM牛脑磷脂酰丝氨酸和0.5μM 1,2-sn二醇甘油。将15μL纯化的PKC(测定中的最终浓度0.38μg/mL)加入孵育混合物中。10分钟后,通过加入30μL酪蛋白30 mg/mL和0.9 mL 12%的三氯乙酸来停止反应[1]。
GSK-3活性测定[3] 测定细胞裂解物和GSK-3β免疫沉淀物中GSK-3的活性。用4 μl抗GSK-3β单克隆抗体和3.75 mg蛋白A-Sepharose在4℃下翻滚2 h,免疫沉淀GSK-3β。免疫沉淀在激酶测定缓冲液(20 mM HEPES, pH 7.5, 20 mM β-甘油磷酸和1 mM EDTA)中洗涤3次,最后在300 μl含有0.1%巯基乙醇和2.5 μM camp依赖性蛋白激酶抑制剂肽(IP20)的激酶测定缓冲液中重悬。用合成肽底物RRAAEELDSRAGS(P)PQL (0.71 mg/ml)[14]分别在20 μl细胞裂解液或20 μl GSK-3β免疫沉淀物中测定GSK-3的活性,无论是否存在GSK-3抑制剂氯化锂(50 mM)。在P81离子交换纸上标记[γ- 32P]ATP孵育15分钟后终止实验。用0.6%的磷酸洗涤4次,用闪烁计数法测定结合放射性。脂肪细胞裂解物和GSK-3β免疫沉淀物对多肽的磷酸化作用基本上被氯化锂完全抑制。提取物中GSK-3的平均活性为1220±144 pmol肽磷酸化/min/g脂肪细胞干重(n=11)。免疫沉淀物中GSK-3β的平均活性为276±54 pmol肽磷酸化/min/g脂肪细胞干重(n=11)。 |
| 细胞实验 |
研究者进一步确定了GF 109203X在两种细胞模型中的效力和特异性:人血小板和瑞士3T3成纤维细胞。GF 109203X有效地阻止血小板中Mr=47000蛋白和Swiss 3T3细胞中Mr=80000蛋白的PKC介导的磷酸化。相反,在相同的模型中,PKC抑制剂未能阻止PKC非依赖性磷酸化。GF 109203X抑制胶原和α-凝血酶诱导的血小板聚集以及胶原触发的ATP分泌。然而,ADP依赖性可逆聚集没有改变。在瑞士3T3成纤维细胞中,GF 109203X逆转了佛波醇12,13-丁酸诱导的表皮生长因子结合的抑制作用,并阻止[3H]胸苷掺入DNA,只有当这是由激活PKC的生长促进剂引起时。我们的结果说明了GF 109203X作为研究PKC参与信号转导途径的工具的潜力[1]。
肿瘤细胞的微囊泡(MV)释放影响药物保留,有助于癌症耐药。策略性地调节MV释放可能会增加癌细胞内的药物保留,并允许降低化疗药物的剂量。外泌体对药物潴留的作用尚不清楚。潜在的外泌体和MV (EMV)生物发生抑制剂,在人前列腺癌(PC3)细胞上测试了其抑制EMV释放的能力,也在PC3和MCF-7(乳腺癌)细胞上测试了其改善化疗的能力。在维持细胞活力的同时,抑制EMV释放最显著的药物是氯脒(Cl-amidine;50µM)和双吲哚马来酰亚胺i(10µM)。凋亡介导的化疗药物5 -氟尿嘧啶(研究者用)在生物细胞显著增强这两种EMV抑制剂的存在,导致62% (Cl-amidine +研究者用)和59% (GF 109203X/bisindolylmaleimide-I +研究者用)减少数量的可行的生物相比,研究者用仅24 h后,MCF-7细胞有相似的增加减少可行的细胞相比,研究者用治疗仅从67% (Cl-amidine +研究者用)到58% (bisindolylmaleimide-I +研究者用)。通过联合治疗,这两种EMV抑制剂进一步减少了活的癌细胞数量。两种抑制剂均不影响细胞活力。联合选定的EMV抑制剂可能是一种提高化疗药物介导的细胞凋亡疗效的新策略。[4] |
| 动物实验 |
Animal/Disease Models: Quinpirole-treated shrews[2]
Doses: 0-20 mg/kg Route of Administration: ip Experimental Results: diminished quinpirole-injection) diminished the mean frequency of quinpirole-induced vomiting in shrews[ 6]. Induces vomiting. Blocks quinpirole-mediated ERK1/2 phosphorylation in the shrew brainstem. In the typical neurovascular coupling experiment, 1 hour after the anesthesia switch from isoflurane to fentanyl, and regular aCSF suffusion (equilibration period), the rats were subjected to one or two sciatic nerve stimulation episodes followed by NS1619 (10 and 50 µM) or K+ (KCl 6 and 12 mM) suffusions under the cranial window for 5 min at each concentration. In the DM and ND groups, after a recovery period of at least 5 min, we initiated a suffusion of GF 109203X(also known as Bisindolylmaleimide I or Gö 6850). GF109203X, at 20 nM, tends to favor the inhibition of PKC-α (IC50 of 8 nM). However, such concentration is thought to inhibit also PKC-βI, -βII and -γ (IC50 of 18, 20, and 21 nM, respectively), but not other PKC isoforms, e.g. δ, ε and ζ (IC50 of 210, 132 and 5800 nM, respectively). Therefore, all the conventional PKC isoforms might be similarly affected by GF109203X. Pial arteriolar diameter changes, relative to baseline, after 40 min suffusion of GF109203X (20 nM) under the cranial window were modest and not significantly different when comparing non-diabetic and diabetic rats (-2 ± 3% and -3 ± 6 %, respectively; P > 0.05). Forty minutes later, a second sciatic nerve stimulation was imposed, followed by re-evaluation of NS1619 and K+-induced dilations. [2] Seven-week-old male C57BL/6 N mice weighing 19–23 g were fed for 1 week to allow them to adapt to the environment. The mice were kept in a constant temperature of 25 °C under a 12-hour light/dark cycle and were fed a standard diet of pellets and water. To determine the relationship between PKC activation and NLRP3, the mice were randomly divided into the following four groups (n = 6 in each group): control (C) group, GF 109203X/bisindolylmaleimide I-pretreated (0.02 mg/kg)(B) group, mechanical ventilation (MV) group and bisindolylmaleimide I-pretreated (1 h) and mechanically ventilated (B + MV) group. Mechanical ventilation was not conducted in C and B groups. The other two groups were mechanically ventilated for 4 h using an ALC-V8 animal ventilator. The ventilation parameters were set as follows: tidal volume 30 ml/kg, respiratory rate 60 times/min, I/E ratio of 1:2, no positive end-expiratory pressure, fraction of inspired oxygen 21% and room temperature 25 °C.[5] |
| 参考文献 |
|
| 其他信息 |
Bim-1 is a member of indoles.
In conclusion, the dysregulation of cerebral perfusion accompanying chronic hyperglycemia, in rat models of type 1 diabetes mellitus, is well-documented. The above pathophysiologic process has been shown to include loss of BKCa and Kir channel-mediated vasodilating functions as well as a significant decrease in the pial arteriolar dilations evoked by somatosensory activation, via sciatic nerve stimulation, in streptozotocin-treated diabetic rats. Present results also suggested that the restoration and maintenance of normoglycemia, via the endogenous production of insulin from transplanted pancreatic islets, are able to prevent the compromise of neurovascular coupling observed in diabetic animals. Moreover, the acute topical administration of an inhibitor of the classical PKC isoforms, GF109203X, was capable of reversing the diabetic cerebrovascular impairments. Both of these neuroprotective mechanisms might have significant clinical implications.[2] Several groups have reported that bisindolylmaleimide IX activates JNK in cells in a PKC-independent manner. Activation of JNK by insulin is blocked by wortmannin in CHO cells expressing the insulin receptor and is likely, therefore, to be downstream of PI3 kinase activation. This raises the possibility that inhibition of GSK-3 activity may lead, presumably indirect, to the activation of JNK. This hypothesis is consistent with the observation that the bisindolylmaleimide IX- and insulin-stimulated JNK activation in rat adipocytes are not additive. It requires rigorous testing, particularly as bisindolylmaleimide IX is known to inhibit other protein kinases, such as MAPKAP kinase and p70S6 kinase. However, it should be noted that these particular kinases are unlikely to be involved as insulin and bisindolylmaleimide IX have opposite effects on their activity. One of the substrates of JNK is c-Jun, which forms part of the activating protein-1 complex (AP-1 complex), and is phosphorylated by JNK on two regulatory sites Ser-63 and Ser-73. Phosphorylation of these sites transactivates c-Jun, and may also explain the increased c-jun expression induced by bisindolylmaleimide IX. Stimulation of AP-1 activity in response to bisindolylmaleimide IX is likely, therefore, to be the result of increased c-Jun synthesis and/or phosphorylation of c-Jun on Ser-63 and Ser-73 by increased JNK activity. However, GSK-3 phosphorylates c-Jun on three sites in a region proximal to the DNA-binding domain (residues 227–252), resulting in decreased c-Jun DNA binding and transcriptional activity. Indeed, transfection experiments have shown that AP-1 activity is inhibited by co-expression of GSK-3. Inhibition of GSK-3 activity by bisindolylmaleimide IX might therefore abolish this negative restraint, thereby increasing c-Jun/AP-1 activity. In summary, we have demonstrated that both bisindolylmaleimide I and IX are potent and direct inhibitors of GSK-3. Our results raise the possibility that some of the insulin-like effects of bisindolylmaleimide IX, in particular the activation of glycogen synthase, may be the result of the ability of these compounds to inhibit GSK-3.[3] |
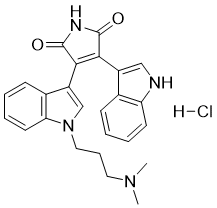
| 分子式 |
C25H24N4O2.HCL
|
|---|---|
| 分子量 |
448.9446
|
| 精确质量 |
448.167
|
| 元素分析 |
C, 66.88; H, 5.61; Cl, 7.90; N, 12.48; O, 7.13
|
| CAS号 |
176504-36-2
|
| 相关CAS号 |
Bisindolylmaleimide I;133052-90-1
|
| PubChem CID |
6419775
|
| 外观&性状 |
Orange to red solid powder
|
| LogP |
4.719
|
| tPSA |
73.62
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
748
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
XRAMWNCMYJHGGH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H24N4O2.ClH/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20;/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31);1H
|
| 化学名 |
3-[1-[3-(Dimethylamino)propyl]indol-3-yl]-4-(1H-indol-3-yl)pyrrole-2,5-dione Hydrochloride
|
| 别名 |
BIS-I HCl; GF-109203X HCl; GO-6860 HCl; 176504-36-2; Bisindolylmaleimide I HCl; Bisindolylmaleimide I, HCl; Bisindolylmaleimide I (hydrochloride); Bisindolylmaleimide I, Hydrochloride; 1H-Pyrrole-2,5-dione, 3-[1-[3-(dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-, hydrochloride (1:1); GF 109203X hydrochloride; DTXSID00423558; BISI HCl; GF109203X HCl; GO6860 HCl; BIS I HCl; GF 109203X HCl; GO 6860 HCl;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~62.5 mg/mL (~139.22 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (4.63 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2275 mL | 11.1373 mL | 22.2747 mL | |
| 5 mM | 0.4455 mL | 2.2275 mL | 4.4549 mL | |
| 10 mM | 0.2227 mL | 1.1137 mL | 2.2275 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。