| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| Other Sizes |

| 靶点 |
PD-1/PD-L1 interaction (IC50 = 1.4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
BMS-1166 是一种强效 PD-1/PD-L1 相互作用抑制剂,在均相时间分辨荧光结合测定中 IC50 为 1.4 nM [1]。 BMS-1166 可以抵消 PD-1/PD-L1 免疫检查点对 T 细胞激活的抑制作用。 BMS-1166 可剂量依赖性地消除 sPD-L1 对 EC 激活的抑制作用[2]。
BMS-1001和BMS-1166可拮抗PD-1/PD-L1免疫检查点对T细胞活化的抑制作用。 BMS-1001和BMS-1166可拮抗可溶性PD-L1对T细胞的抑制作用。 BMS-1001和BMS-1166在溶液中诱导PD-L1二聚化。BMS-1001和BMS-1166显著改善了细胞毒性,允许使用更高的浓度。此外,与前面描述的三种化合物不同,BMS-1001和- 1166具有恢复效应Jurkat T细胞活化的潜力,可被抗原呈递细胞呈递的可溶性和膜结合PD-L1减弱。[2] |
| 体内研究 (In Vivo) |
NP19 [[BMS-1166类似物]]在H22肝癌小鼠模型中的体内抗肿瘤活性[3]
鉴于NP19在黑色素瘤B16-F10肿瘤模型中具有良好的体内抗肿瘤效果,且PD-1/PD-L1抑制剂具有广谱的抗肿瘤活性,我们进一步利用H22肝癌模型BALB/c小鼠对复方NP19的体内抗肿瘤效果进行了评价。每只小鼠右侧皮下注射80万个H22细胞。当肿瘤体积达到约100 mm3时,随机选取小鼠,通过腹腔注射NP19或载体溶液治疗14天。如图8所示,NP19在25 mg/kg剂量下具有显著的体内抗肿瘤功效,TGI为76.5%(图8A, 8B, 8C)。此外,NP19没有引起明显的体重减轻(图8D),表明该化合物耐受性良好。 NP19 [[BMS-1166类似物]]在B16-F10小鼠黑色素瘤模型中的体内抗肿瘤活性[3] 为了确定新合成化合物的体外抗pd -1/PD-L1活性是否可以转化为体内功效,我们在小鼠黑色素瘤B16-F10肿瘤模型上测试了化合物NP19的抗肿瘤活性。选择NP19进行体内疗效研究,是因为与更有效的化合物NP2或同样有效的化合物NP12相比,NP19易于合成且细胞毒性较小(表9)。我们将携带黑色素瘤的BALB/c小鼠分别以载药对照和NP19 (25 mg/kg、50 mg/kg、100 mg/kg)灌胃治疗,每天1次,持续15天。如图6所示,治疗15天后,NP19治疗显著抑制了黑色素瘤的生长。 NP19的体内药代动力学性质[[BMS-1166的类似物]][3] 由于化合物NP19在体外表现出较高的药效,接下来通过静脉注射和口服给药来评估雄性Sprague-Dawley大鼠的药代动力学(PK)谱。表8总结了关键po和静脉给药PK参数。单次静脉给药1 mg/kg化合物NP19后,NP19的半衰期(t1/2)为1.5±0.5 h,清除率(CL)为0.9±0.2 L/h/kg,表观分布体积(Vss)为2.1±0.5 L/kg。NP19口服给药剂量为10 mg/kg时,观察到NP19的口服吸收(Tmax = 0.6±0.2 h)、长半衰期(t1/2 = 10.9±7.7 h)和口服生物利用度(F = 5%)。此外,在大鼠中未观察到明显的不良反应。NP19经口服灌胃后的半衰期(10.9 h)比静脉注射的半衰期(1.5 h)长得多;这可能是由于NP19的高亲脂性(logP = 7.9)或水溶性差。结果,NP19表现出翻转式药代动力学。这种翻转式的药代动力学有时会发生在水溶性较差的化合物中,如利巴米胺(43),由于水溶性较差(7.6 μg/mL),其t1/2 (p.o)/t1/2 (i.v)比为13.5。另一个例子是李建明等(44)报道的亲脂化合物IAT(一种水溶性为19 μg/mL的抗微管蛋白剂),其t1/2 (p.o.)/t1/2 (i.v.)的比值为~ 5,类似于NP19 [t1/2 (p.o.)/t1/2 (i.v.) = 7.1]。由于化合物NP19的口服生物利用度较低,我们推测需要高剂量才能提供足够的药物浓度以显示抗肿瘤功效。因此,我们进一步研究了化合物NP19的体内活性。 |
| 酶活实验 |
体外PD-1/PD-L1结合试验[3]
利用PD-1/PD-L1均质时间分辨荧光(HTRF)结合实验研究了化合物抑制PD-1/PD-L1相互作用的能力。PD-1/PD-L1结合检测试剂盒购自Cisbio。实验按照说明书进行,说明书可从https://www.cisbio.com/usa/drug-discovery/human-pd1pd-l1-biochemical-interaction-assay下载。 BMS 最近公开了第一个针对 PD-1/PD-L1 通路的非肽类小分子抑制剂,该抑制剂在均相时间分辨荧光 (HTRF) 结合测定中显示出活性;但没有提供支持其活动的进一步数据。 核磁共振测量[2] 在以15NH4Cl为唯一氮源的M9最小培养基中表达蛋白,获得均匀的15N标记。对于核磁共振测量,缓冲液通过凝胶过滤交换到pH为7.4 (PD-L1)的PBS或含有100 mM NaCl pH为6.4 (PD-1)的25 mM磷酸钠。在样品中加入10% (v/v)的D2O来提供锁定信号。所有光谱在300K下使用Bruker Avance III 600 MHz光谱仪记录。化合物与PD-L1的相互作用通过监测1h - 15n2d HMQC中核磁共振化学位移的扰动来评估。测试化合物解离PD-L1/PD-1的能力使用拮抗剂诱导解离试验进行评估。简而言之,15n标记的PD-1 (0.2 mM)与未标记的PD-L1稍微过滴定。这些化合物被合并到所得到的混合物中。实验过程中,h - 15n信号由HMQC监测。 PD1/PD-L1检查点试验[2] 检测前24小时,将aAPCs以每孔10 000个的密度在培养基中接种于96孔白色板中。在检测当天,在含有1% FBS的RPMI 1640中制备3.5倍连续稀释的抗体。在DMSO中制备了一系列BMS化合物[BMS-1166],并在含有1% FBS的RPMI 1640中配制。通过这种方法,所有样品中DMSO的浓度保持不变。从孔中取出95 μl的培养基,用40 μl的复合稀释液覆盖细胞。在含有1% FBS的40 μl RPMI 1640溶液中,每孔加入2万个ECs。37℃孵育6 h后,室温平衡10 min,每孔加入80 μl Bio-Glo试剂。孵育10分钟后,用FlexStation 3定量荧光。用Hill方程拟合实验数据,确定了一半最大有效浓度(EC50)和最大发光值(RLUmax)。 PD-1/ pd - l1效应试验[2] 为了评估BMS对可溶性PD-L1抑制T细胞的影响,在重组人PD-L1存在的情况下,用抗cd3抗体刺激ECs。为此,用5 μg/ml的抗cd3抗体或PBS中的同型对照液在96孔白色平底板上涂覆过夜,温度为4℃。除去抗体溶液,用PBS洗涤3次并干燥。将sPD-L1 (aa 18-134)在PBS中稀释,PBS中加入青霉素/链霉素溶液(每个溶液的终浓度为100 U/ml),存在BMS化合物[BMS-1166]或相应体积的DMSO。然后在抗体包被板的每孔中加入15 μl的溶液。ECs离心后稀释至5万/ ml,每孔加入细胞液60 μl。sPD-L1终浓度为10 μg/ml (0.6 μM)。BMS化合物[BMS-1166]的最终浓度分别为:0.12、0.3、1.2和3 μM,得到BMS:sPD-L1的摩尔比分别为1:5、1:2、2:1和5:1。细胞培养24小时,根据制造商的说明,使用Bio-Glo荧光素酶测定系统进行荧光素酶活性测定。 |
| 细胞实验 |
细胞系[2]
为了验证BMS化合物[BMS-1166]抑制PD-1/PD-L1相互作用的效力,使用了基于细胞的PD-1/PD-L1免疫检查点阻断模型。在实验中,使用了两种模型细胞系:人工抗原呈递细胞(PD-L1+ aAPC/CHO-K1细胞,称为aAPCs)过表达TCR配体和PD-L1, T细胞替代品,一种经过修饰的过表达PD-1的Jurkat T细胞系,在NFAT启动子(PD-1效应细胞,称为ECs)的控制下携带荧光素酶报告细胞。从Promega中获得细胞,在添加10%胎牛血清、100 U/ml青霉素和100 U/ml链霉素的RPMI 1640培养基中培养。此外,细胞在持续存在的潮霉素B (50 μg/ml)和G418 (250 μg/ml)中繁殖,以提供引入的遗传构建物的稳定表达。后两种抗生素在实验中被省略。流式细胞术证实了ECs上PD-1和aAPCs上PD-L1的过表达(未示出),通过监测抗cd3抗体刺激后的荧光素酶活性证实了荧光素酶表达基因的存在。抗生素选择、流式细胞术和报告基因表达作为细胞系鉴定方法。使用基于pcr的方法对细胞进行周期性检测,发现支原体污染呈阴性。 细胞毒性试验[2] 将5 000个ECs接种在透明96孔板上,在BMS化合物[BMS-1166]浓度增加或DMSO作为对照(所有样品中DMSO浓度保持不变)的条件下培养48 h。处理后,根据制造商的说明,使用Biolog氧化还原染料混合MB进行代谢活性测试。 流式细胞术测定[2] 流式细胞术检测sPD-L1 (aa 18-134)与ECs的结合情况。将his标记的PD-L1蛋白或其突变体用NTA-Atto 647 N荧光染料在22°C下以8:1的摩尔比(蛋白:染料)染色2小时。将PD-L1-Atto与所测化合物或抗体在150 μl PBS中配制。样品在4°C黑暗中孵育30分钟。同时,将ECs离心,PBS洗涤,并以1 × 106个细胞/ml的浓度悬浮在新鲜PBS中,每个样品中加入50 μl ECs,冰孵育60 min,最终成分浓度为:PD-L1 (1.5 μM) 25 μg/ml,抗PD-L1抗体和对照抗体125 μg/ml, BMS化合物1 μM [BMS-1166]。使用BD FACS Verse流式细胞仪和BD FACSuite v1.0.6软件对样本进行分析。 |
| 动物实验 |
Pharmacokinetic Study in Male Sprague–Dawley Rats[3]
Male Sprague–Dawley rats (200–220 g) were used to study the pharmacokinetics of compound NP19 [an analog of BMS-1166]. Diet was prohibited for 12 h before the experiment, but water was freely available. Blood samples (0.3 mL) were collected from the tail vein into heparinized 1.5 mL polythene tubes at 0.0833, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after oral (10 mg/kg) or intravenous (1 mg/kg) administration of compound NP19. The compound was dissolved in 5% DMSO and 95% PEG-300 for intravenous administration or suspended in 0.5% sodium carboxymethyl cellulose (CMC-Na) for oral administration. The samples were immediately centrifuged at 3000g for 10 min. The plasma as-obtained (100 μL) was stored at −20 °C until analysis. PK parameters were determined from individual animal data using noncompartmental analysis in DAS (Drug and statistics) software. Instruments and analytical conditions for PK studies: A UPLC-MS/MS system with ACQUITY I-Class UPLC and a XEVO TQD triple quadrupole mass spectrometer (Waters Corp., Milford, MA, USA), equipped with an electrospray ionization (ESI) interface, was used to analyze the blood samples. The UPLC system was comprised of a Binary Solvent Manager (BSM) and a Sample Manager with Flow-Through Needle (SM-FTN). Masslynx 4.1 software (Waters Corp.) was used for data acquisition and instrument control. Multiple reaction monitoring (MRM) modes of m/z 555.35 → 181.03 for NP19 and m/z 237 → 194.1 for carbamazepine were utilized to conduct quantitative analysis. In Vivo Efficacy Study in Mouse B16F10 Melanoma Model[3] BALB/c mice, aged 6–8 weeks old, were used to study the inhibition effect of NP19 [an analog of BMS-1166]on subcutaneous transplanted model of melanoma cells. Murine B16F10 melanoma cells growing in a logarithmic growth phase were suspended in PBS at a density of 2 × 106 per mL. Each mouse was inoculated subcutaneously with 200 μL containing 4 × 105 cells. After tumors reached approximately 100 mm3 in volume, mice were divided into four groups randomly (n = 10) and treated with NP19 (25, 50, 100 mg/kg) and vehicle, respectively. The drugs were administered via intragastric gavage once a day for 15 days. The vehicle group was administered with 0.5% sodium carboxymethyl cellulose (CMC-Na). Animal activity and body weight were monitored during the entire experiment period to assess acute toxicity. Mice were sacrificed 16 days after the initiation of the treatment, and the tumor tissue and major organ (liver, spleen, thymus, and kidney) samples were collected. The harvested tumor tissue and organs (liver, kidney) were fixed in 4% paraformaldehyde, processed into paraffin routinely, stained with hematoxylin and eosin (H&E), and captured by microscope. Tumor growth inhibition value (TGI) was calculated using the formula: TGI(%) = [1 – Wt/Wv] × 100%, where Wt and Wv are the mean tumor weight of treatment group and vehicle control. In Vivo Efficacy Study in Mouse H22 Hepatoma Tumor Model[3] 6–8 weeks old male BALB/c mice were used. A total of 8 × 105 H22 cells were inoculated into the right flank of each mouse according to protocols of tumor transplant research. NP19 [an analog of BMS-1166]was dissolved in 5% DMSO, 40% PEG-200 and 55% saline solution to produce desired concentrations. Mice in control groups were injected intraperitoneally with 200 μL of vehicle solution only. Tumor volume was measured every 2 days with a traceable electronic digital caliper and calculated using the formula a × b2 × 0.5, where a and b represented the larger and smaller diameters, respectively. The mice were sacrificed after the treatments and tumors were excised and weighed. |
| 参考文献 |
|
| 其他信息 |
Cancer immunotherapy based on antibodies targeting the immune checkpoint PD-1/PD-L1 pathway has seen unprecedented clinical responses and constitutes the new paradigm in cancer therapy. The antibody-based immunotherapies have several limitations such as high production cost of the antibodies or their long half-life. Small-molecule inhibitors of the PD-1/PD-L1 interaction have been highly anticipated as a promising alternative or complementary therapeutic to the monoclonal antibodies (mAbs). Currently, the field of developing anti-PD-1/PD-L1 small-molecule inhibitors is intensively explored. In this paper, we review anti-PD-1/PD-L1 small-molecule and peptide-based inhibitors and discuss recent structural and preclinical/clinical aspects of their development. Discovery of the therapeutics based on small-molecule inhibitors of the PD-1/PD-L1 interaction represents a promising but challenging perspective in cancer treatment.[1]
Antibodies targeting the PD-1/PD-L1 immune checkpoint achieved spectacular success in anticancer therapy in the recent years. In contrast, no small molecules with cellular activity have been reported so far. Here we provide evidence that small molecules are capable of alleviating the PD-1/PD-L1 immune checkpoint-mediated exhaustion of Jurkat T-lymphocytes. The two optimized small-molecule inhibitors of the PD-1/PD-L1 interaction, BMS-1001 and BMS-1166, developed by Bristol-Myers Squibb, bind to human PD-L1 and block its interaction with PD-1, when tested on isolated proteins. The compounds present low toxicity towards tested cell lines and block the interaction of soluble PD-L1 with the cell surface-expressed PD-1. As a result, BMS-1001 and BMS-1166 alleviate the inhibitory effect of the soluble PD-L1 on the T-cell receptor-mediated activation of T-lymphocytes. Moreover, the compounds were effective in attenuating the inhibitory effect of the cell surface-associated PD-L1. We also determined the X-ray structures of the complexes of BMS-1001 and BMS-1166 with PD-L1, which revealed features that may be responsible for increased potency of the compounds compared to their predecessors. Further development may lead to the design of an anticancer therapy based on the orally delivered immune checkpoint inhibition.[2] Blockade of the PD-1/PD-L1 immune checkpoint pathway with monoclonal antibodies has provided significant advances in cancer treatment. The antibody-based immunotherapies carry a number of disadvantages such as the high cost of the antibodies, their limited half-life, and immunogenicity. Development of small-molecule PD-1/PD-L1 inhibitors that could overcome these drawbacks is slow because of the incomplete structural information for this pathway. The first chemical PD-1/PD-L1 inhibitors have been recently disclosed by Bristol-Myers Squibb. Here we present NMR and X-ray characterization for the two classes of these inhibitors. The X-ray structures of the PD-L1/inhibitor complexes reveal one inhibitor molecule located at the center of the PD-L1 homodimer, filling a deep hydrophobic channel-like pocket between two PD-L1 molecules. Derivatives of (2-methyl-3-biphenylyl)methanol exhibit the structures capped on one side of the channel, whereas the compounds based on [3-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-methylphenyl]methanol induce an enlarged interaction interface that results in the open "face-back" tunnel through the PD-L1 dimer.[3] |
| 分子式 |
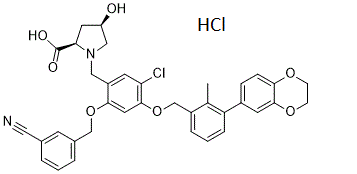
C36H34CL2N2O7
|
|
|---|---|---|
| 分子量 |
677.570368289948
|
|
| 精确质量 |
676.174
|
|
| 元素分析 |
C, 63.82; H, 5.06; Cl, 10.46; N, 4.13; O, 16.53
|
|
| CAS号 |
2113650-05-6
|
|
| 相关CAS号 |
BMS-1166;1818314-88-3
|
|
| PubChem CID |
139035005
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| tPSA |
122
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
47
|
|
| 分子复杂度/Complexity |
1060
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
ClC1=C(C=C(C(=C1)CN1C[C@@H](C[C@@H]1C(=O)O)O)OCC1C=CC=C(C#N)C=1)OCC1C=CC=C(C2C=CC3=C(C=2)OCCO3)C=1C.Cl
|
|
| InChi Key |
HYWQPFPFYSOTHD-UVFMYQNSSA-N
|
|
| InChi Code |
InChI=1S/C36H33ClN2O7.ClH/c1-22-26(6-3-7-29(22)25-8-9-32-35(14-25)44-11-10-43-32)21-46-34-16-33(45-20-24-5-2-4-23(12-24)17-38)27(13-30(34)37)18-39-19-28(40)15-31(39)36(41)42;/h2-9,12-14,16,28,31,40H,10-11,15,18-21H2,1H3,(H,41,42);1H/t28-,31-;/m1./s1
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.69 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.69 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4759 mL | 7.3793 mL | 14.7586 mL | |
| 5 mM | 0.2952 mL | 1.4759 mL | 2.9517 mL | |
| 10 mM | 0.1476 mL | 0.7379 mL | 1.4759 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Structures and the PD-1/PD-L1 blocking potential of BMS compounds.Oncotarget.2017Aug 7;8(42):72167-72181. |
|---|
 Cytotoxicity and activity of BMS compounds in PD-1/PD-L1 checkpoint assay.Oncotarget.2017Aug 7;8(42):72167-72181. |
 BMS compounds restore the sPD-L1-supressed activation of Jurkat T-cells.Oncotarget.2017 |
 BMS-1166 induces binding cleft opening.Oncotarget.2017Aug 7;8(42):72167-72181. |
|---|
 Decomposition of BMS-1166.Oncotarget.2017Aug 7;8(42):72167-72181. |
 he prediction of BMS-1001 and −1166 binding sites on PD-L1 surface.Oncotarget.2017Aug 7;8(42):72167-72181. |