| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
体外活性:布林佐胺是已成功开发并上市的最新外用 CAI。是一种安全有效的青光眼药物。在体外测定中,布林佐胺对 CA-II 具有最高的亲和力(Ki 为 0.13nM)和抑制效力(IC50 为 3.19 nM)。与 CA-I 和 CAIV 相比,它对 CA-II 具有更高的亲和力和更强的效力。在体内模型中,给予布林佐胺可显着降低氩激光小梁成形术引起的高眼压兔和食蟹猴的眼压(IOP)。激酶测定:Brinzolamide(AL 4862) 是一种有效的碳酸酐酶 II 抑制剂,IC50 为 3.19 nM。
|
|---|---|
| 体内研究 (In Vivo) |
在血压正常的 NZW 兔中,植入硅胶基质中的布林佐胺(7.5 毫克或 12 毫克)具有令人难以置信的良好耐受性,导致药物释放时间延长,眼压 (IOP) 显着下降长达 28 天[2]。没有观察到负面影响或毒性症状。布林佐胺在家兔体内的药代动力学参数[1]。局部给药 (500 mg) 局部给药 (500 mg) PK 参数 房水虹膜-睫状体 房水虹膜-睫状体 Tmax (h) 0.08 0.5 1 0.25 Cmax (ng/mL, ng/g) 11,050 1964 408 1245 终点 t1 /2 (h) 3.4 13 2 13.6 AUC0-24h (h*ng/mL, h*ng/g) 17,780 7725 1896 11414 AUC0-∞ (h*ng/mL, h*ng/g) 17,836 8839 1955 16628 剂量-归一化 AUC0-∞ (h*/mL, h*/g) 4 2 0.004 0.03
|
| 动物实验 |
Animal/Disease Models: Rabbits [2]
Doses: 7.5 mg, 12 mg Route of Administration: Brinzolamide silicone matrix implant placed in the episcleral space Experimental Results: Resulted in a significant IOP reduction of 4.6 mmHg on days 10-28, with concentrations of 12 mg. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Brinzolamide is absorbed through the cornea following topical ocular administration. The substance is also absorbed into the systemic circulation where it binds strongly to carbonic anhydrase in red blood cells (RBCs). Plasma concentrations are very low. Brinzolamide is eliminated predominantly in the urine as unchanged drug.N-Desethyl brinzolamide is also found in the urine along with lower concentrations of the N-desmethoxypropyl and O-desmethyl metabolites. Metabolism / Metabolites Brinzolamide is metabolized by hepatic cytochrome P450 isozymes, specifically CYP3A4, CYP2A6, CYP2B6, CYP2C8 and CYP2C9. The primary metabolite is N-desethylbrinzolamide followed by the N-desmethoxypropyl and O-desmethyl metabolites as well as an N-propionic acid analog formed by oxidation of the N-propyl side chain of O-desmethyl brinzolamide. Brinzolamide and N-desethylbrinzolamide do not inhibit cytochrome P450 isozymes at concentrations at least 100-fold above maximum systemic levels. Brimonidine is extensively metabolized by hepatic aldehyde oxidase with the formation of 2-oxobrimonidine, 3-oxobrimonidine, and 2,3-dioxobrimonidine being the major metabolites. Oxidative cleavage of the imidazoline ring to 5-bromo-6-guanidinoquinoxaline is also observed. Biological Half-Life Due to its affinity for CAII, brinzolamide distributes extensively into the red blood cells (RBCs) and exhibits a long half-life in whole blood (approximately 111 days). |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of brinzolamide ophthalmic drops during breastfeeding. French guidelines recommend ophthalmic carbonic anhydrase inhibitor drops such as brinzolamide as a preferred therapy for glaucoma during breastfeeding. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binding to plasma proteins is approximately 60%. |
| 参考文献 |
|
| 其他信息 |
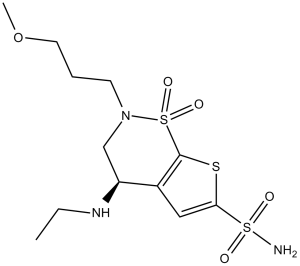
Brinzolamide is a sulfonamide and a thienothiazine. It has a role as an antiglaucoma drug and an EC 4.2.1.1 (carbonic anhydrase) inhibitor.
Brinzolamide is a highly specific, non-competitive, reversible carbonic anhydrase II (CA-II) inhibitor indicated to reduce ocular pressure in patients with ocular hypertension or open-angle glaucoma. Although the exact pathophysiology of glaucoma is still unknown, one of the main hallmarks of this disease is vascular dysregulation and abnormalities. The resulting vascular resistance increases intraocular pressure, thus impairing ocular perfusion. Although systemic anti-carbonic anhydrase (CA) therapy has been used for almost 50 years with varying degrees of success, systemic administration results in an increase in incidences of adverse effects. Brinzolamide was developed as a topical solution to the systemic side effects and [dorzolamide], the first-ever approved topical CA inhibitor with contrasting results and evidence. Unlike [dorzolamide], brinzolamide has a higher lipophilicity to facilitate diffusion across the blood-retinal barrier. Brinzolamide was approved by the FDA in 1998 as a standalone product and in 2013 as a combination product with brimonidine tartrate. In Europe, it was also approved as a combination product with timolol in 2008. Brinzolamide is a Carbonic Anhydrase Inhibitor. The mechanism of action of brinzolamide is as a Carbonic Anhydrase Inhibitor. Brinzolamide is a sulfonamide and carbonic anhydrase inhibitor with specific affinity for carbonic anhydrase II. Following topical ocular administration, brinzolamide inhibits carbonic anhydrase II, an enzyme that is responsible for the movement of sodium and fluid transport in the eye. This inhibition leads to a decrease in aqueous humor secretion, probably by slowing the formation of bicarbonate ions, and results in a reduction in intraocular pressure. Brinzolamide is used to treat increased pressure in the eye caused by open-angle glaucoma. See also: Brimonidine Tartrate; Brinzolamide (component of). Drug Indication Brinzolamide, either as a standalone agent or in combination with brimonidine, is approved by the FDA for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. Brinzolamide is also approved in Europe to be used in combination with timolol to treat the same conditions. FDA Label Azopt is indicated to decrease elevated intraocular pressure in: ocular hypertension; open-angle glaucomaas monotherapy in adult patients unresponsive to beta-blockers or in adult patients in whom beta-blockers are contraindicated, or as adjunctive therapy to beta-blockers or prostaglandin analogues. Mechanism of Action Brinzolamide is a highly specific, reversible, non-competitive inhibitor of carbonic anhydrases (CA), the enzymes catalyzing the reversible reaction of water and carbon dioxide (CO2) to form bicarbonate ions. Although there are 7 isoforms of CA in human tissues, brinzolamide has the highest affinity to CA II. Brinzolamide and its active metabolites were not found to displace any known ligands in vitro from their respective receptors or enzymes commonly involved in producing side effects or ancillary pharmacology, thus explaining brinzolamide's high order of safety. |
| 分子式 |
C12H21N3O5S3
|
|---|---|
| 分子量 |
383.51
|
| 精确质量 |
383.064
|
| CAS号 |
138890-62-7
|
| 相关CAS号 |
Brinzolamide hydrochloride;150937-43-2;Brinzolamide-d5;1217651-02-9
|
| PubChem CID |
68844
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
586.0±60.0 °C at 760 mmHg
|
| 闪点 |
308.2±32.9 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.626
|
| LogP |
-0.65
|
| tPSA |
163.8
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
598
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CCN[C@H]1CN(S(=O)(=O)C2=C1C=C(S2)S(=O)(=O)N)CCCOC
|
| InChi Key |
HCRKCZRJWPKOAR-JTQLQIEISA-N
|
| InChi Code |
InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1
|
| 化学名 |
(4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-3,4-dihydrothieno[3,2-e]thiazine-6-sulfonamide
|
| 别名 |
AL 4862; Brinzolamide; trade names Azopt, Alcon Laboratories, Befardin, Fardi Medicals; AL-4862; AL4862
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 27.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6075 mL | 13.0375 mL | 26.0749 mL | |
| 5 mM | 0.5215 mL | 2.6075 mL | 5.2150 mL | |
| 10 mM | 0.2607 mL | 1.3037 mL | 2.6075 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03896633 | Completed Has Results | Drug: brinzolamide 1% ophthalmic suspension |
Glaucoma Open Angle or Ocular Hypertension |
Bausch & Lomb Incorporated | February 28, 2018 | Phase 1 Phase 2 |
| NCT04523844 | Completed | Drug: Brinzolamide-brimonidine Fixed Combination |
Eye Diseases Injection Complication |
General Hospital of Athens Elpis | May 11, 2020 | Not Applicable |
| NCT03494257 | Completed | Drug: Brinzolamide-Brimonidine fixed combination |
Cataract Intraocular Pressure |
University Hospital of Patras | September 4, 2017 | Not Applicable |
| NCT01721707 | Withdrawn | Drug: Latanoprost+Brinzolamide combination |
Open Angle Glaucoma Ocular Hypertension |
Adapt Produtos Oftalmológicos Ltda. | December 2012 | Phase 3 |
|
|---|
|
|