| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
溴芬酸(0-80 μg/mL;24 小时)以浓度依赖性方式抑制由转化生长因子-β2 引起的 HLEC-B3 上皮-间质转化 [2]。溴芬酸(80 μg/Ml;48 小时)可抑制人前囊中转化生长因子-β2 诱导的上皮细胞向间质细胞的转变 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
在大鼠中,溴芬酸(0.0032-3.16%;100 或 200 μL;涂于背部)在剂量低至 0.1%(治疗前 4 小时)或 0.32%(治疗前 18 小时)时表现出显着的抗菌作用。炎症增加[3]。在大鼠中,应用 100 μL 溴芬酸 (0.032-3.16%) 会产生剂量依赖性抗炎作用 [3]。当直接给予豚鼠暴露于紫外线的皮肤区域时,溴芬酸(0.032-1.0%;50 μL)在预防红斑方面的效果比吲哚美辛高 26 倍 [3]。当将溴芬酸(0.0032-0.1%;50 μL)每天四小时、每周五天给予未注射的大鼠爪子时,大鼠双后肢的爪子体积以剂量和时间依赖性方式减少[3]。当局部给予腹部时,溴芬酸(0.32%;50 μL)可显着防止给予乙酰胆碱的小鼠腹部收缩[3]。当连续四个星期每天使用两次时,每只眼睛使用 1 μL (0.09%) 溴芬酸滴眼液可部分降低角膜染色,四周后这种情况会减慢 [4]。
|
| 细胞实验 |
细胞活力测定[2]
细胞类型:经转化生长因子-β2处理的人前囊 测试浓度: 80 μg/mL 孵育时间:48小时 实验结果:原代LEC中转化生长因子-β2诱导的上皮-间质转化受到抑制。 细胞迁移测定[2] 细胞类型: HLEC-B3 细胞 测试浓度: 0、20、40、60 和80 μg/mL 孵育时间:24小时 实验结果:抑制转化生长因子-β2诱导的细胞迁移HLEC-B3 细胞,并表现出对上皮间质转化标志物过度表达的抑制。 |
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rats (150-250 g) injected with carrageenan [3]
Doses: 0.0032, 0.01, 0.032, 0.1, 0.32, 1.0, 3.16% (100 or 200 μL) Route of Administration: 1 Carrageenan injected on back - 72 hrs (hrs (hours)) before injection Experimental Results: Significant anti-inflammatory activity was produced when applied at 0.32% 1, 2 and 4 hrs (hrs (hours)) before carrageenan challenge. Carrageenan is effective when applied 1 or 4 hrs (hrs (hours)) before challenge, but not 0.2% when applied 24 hrs (hrs (hours)) (or more) before challenge. Animal/Disease Models: Male injected with Salin or BTX-B[4] Doses: 1 μL (0.09%) per eye Route of Administration: eye drops; 1 μL (0.09%) per eye; twice a day; 4-week Experimental Results: Corneal fluorescein staining scores improved 4 weeks after treatment. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The plasma concentration of bromfenac following ocular administration in humans is unknown. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Milk levels of bromfenac are likely to be low with the usual oral dosage, but milk levels have not been measured after higher injectable dosages. Use caution when using bromfenac in nursing mothers, especially with the injectable drug. Maternal use of bromfenac eye drops would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
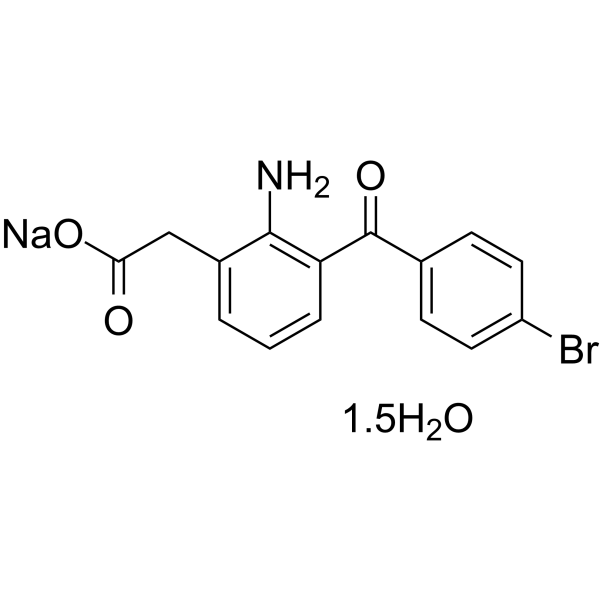
Bromfenac is amfenac in which the the hydrogen at the 4 position of the benzoyl group is substituted by bromine. It is used for the management of ocular pain and treatment of postoperative inflammation in patients who have undergone cataract extraction. It was withdrawn from the US market in 1998, following concerns over off-label abuse and hepatic failure. It has a role as a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is a member of benzophenones, a substituted aniline, an aromatic amino acid and an organobromine compound. It is functionally related to an amfenac. It is a conjugate acid of a bromfenac(1-).
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Ophthalmic NSAIDs are becoming a cornerstone for the management of ocular pain and inflammation. Their well-characterized anti-inflammatory activity, analgesic property, and established safety record have also made NSAIDs an important tool for optimizing surgical outcomes. Non-ophthalmic formulations of bromfenac were withdrawn in the US in 1998 due to cases of severe liver toxicity. Bromfenac is a Nonsteroidal Anti-inflammatory Drug. The mechanism of action of bromfenac is as a Cyclooxygenase Inhibitor. Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and anti-inflammatory activities. Upon ophthalmic administration, bromfenac binds to and inhibits the activity of cyclooxygenase II (COX II), an enzyme which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins (PG). By inhibiting PG formation, bromfenac is able to inhibit PG-induced inflammation, thereby preventing vasodilation, leukocytosis, disruption of the blood-aqueous humor barrier, an increase in vascular permeability and an increase in intraocular pressure (IOP). See also: Bromfenac Sodium (has salt form); Bromfenac; prednisolone acetate (component of) ... View More ... Drug Indication For the treatment of postoperative inflammation in patients who have undergone cataract extraction. FDA Label Treatment of postoperative ocular inflammation following cataract extraction in adults. Mechanism of Action The mechanism of its action is thought to be due to its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed in animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increased vascular permeability, leukocytosis, and increased intraocular pressure. |
| 分子式 |
C30H28BR2N2NA2O9
|
|---|---|
| 分子量 |
766.35
|
| 精确质量 |
333
|
| CAS号 |
120638-55-3
|
| 相关CAS号 |
Bromfenac sodium;91714-93-1;Bromfenac;91714-94-2
|
| PubChem CID |
60726
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
562.2±50.0 °C at 760 mmHg
|
| 闪点 |
293.8±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.663
|
| LogP |
2.72
|
| tPSA |
80.39
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
366
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZBPLOVFIXSTCRZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H12BrNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)
|
| 化学名 |
2-[2-amino-3-(4-bromobenzoyl)phenyl]acetic acid
|
| 别名 |
AHR10282B; AHR 10282B
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~260.98 mM)
H2O : ≥ 100 mg/mL (~260.98 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 33.33 mg/mL (86.98 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3049 mL | 6.5244 mL | 13.0489 mL | |
| 5 mM | 0.2610 mL | 1.3049 mL | 2.6098 mL | |
| 10 mM | 0.1305 mL | 0.6524 mL | 1.3049 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。