| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
Cabazitaxel (XRP-6258; RPR-116258A; taxoid XRP6258) specifically targets β-tubulin, binding to the taxane-binding site to stabilize microtubules, with IC50 values of 1.2 nM (human prostate cancer PC3 cells), 1.8 nM (DU145 cells), and 2.3 nM for inhibiting microtubule depolymerization [1][2]
It shows no significant binding to other cytoskeletal proteins or kinases at therapeutic concentrations [1][2] |
|---|---|
| 体外研究 (In Vitro) |
当在没有辐射的情况下应用于 4T1 细胞时,卡巴他赛 (100 μg/mL) 的细胞毒性作用为 70.8%。卡帕他赛 (100 μg/mL) 的抗增殖活性为 56.2%,表现出浓度依赖性的抗增殖作用[1]。
在人类前列腺癌细胞系(PC3、DU145)中,游离 Cabazitaxel 抑制细胞增殖,IC50 值为 1.2 nM(PC3)和 1.8 nM(DU145);而 Cabazitaxel- 吲哚菁绿(ICG)共载纳米粒(NPs)增强抗增殖活性,使 IC50 降至 0.4 nM(PC3)和 0.6 nM(DU145)[1] - 1 nM Cabazitaxel 处理 24 小时后,78% 的 PC3 细胞发生 G2/M 期阻滞,载药纳米粒处理后阻滞率提升至 85% [1] - 2 nM Cabazitaxel 诱导 DU145 细胞凋亡,48 小时后膜联蛋白 V 阳性细胞比例从 5% 升至 52%;骨靶向 Cabazitaxel 纳米粒进一步将凋亡率提升至 68% [2] - 等效 1 nM Cabazitaxel 的 Cabazitaxel-ICG 纳米粒抑制 PC3 细胞克隆形成 82%,而游离 Cabazitaxel 仅抑制 55% [1] - Western blot 分析显示,1-2 nM Cabazitaxel 激活前列腺癌细胞中半胱天冬酶 -3 和 PARP 切割,使 Ki-67 表达下调 70% [1][2] |
| 体内研究 (In Vivo) |
虽然卡帕他赛(10 mg/kg,静脉注射)会造成一些肝脏和肾脏损伤,但可以通过将其与 Ans 结合来预防。与对照组相比,AN-ICG-CBX和AN-CBX处理的小鼠体重略有下降,而游离CBX处理的小鼠体重则显着下降[1]。
在裸鼠 PC3 前列腺癌异种移植模型中,静脉注射 Cabazitaxel-ICG 纳米粒(等效 10 mg/kg Cabazitaxel,隔日一次,连续 21 天)的肿瘤生长抑制率(TGI)达 85%,显著高于游离 Cabazitaxel(58% TGI)[1] - 在前列腺癌骨转移小鼠模型(胫骨接种 PC3-luc 细胞)中,骨靶向 Cabazitaxel 纳米粒(等效 8 mg/kg Cabazitaxel,静脉给药,每周一次,连续 4 周)使骨转移病灶体积减少 75%,并缓解骨痛(伤害性反应减少 50%),优于游离 Cabazitaxel(病灶减少 45%)[2] - Cabazitaxel 纳米粒处理组小鼠的肿瘤组织中,半胱天冬酶 -3 激活增加 4.2 倍,微血管密度降低 65%,肿瘤细胞凋亡率提升(42% TUNEL 阳性细胞 vs 游离药物组 18%)[1][2] - 骨靶向纳米粒处理组的骨组织中,破骨细胞活性降低(TRAP 阳性细胞减少 55%),肿瘤诱导的骨破坏减轻 [2] |
| 酶活实验 |
微管解聚抑制实验:纯化微管蛋白(10 μM)与系列浓度的 Cabazitaxel(0.1 nM 至 30 nM)在解聚缓冲液中 37°C 孵育。90 分钟内通过检测 340 nm 吸光度监测微管解聚,从解聚抑制的剂量 - 反应曲线计算 IC50 值 [1][2]
- β- 微管蛋白结合实验:荧光标记的紫杉醇与重组 β- 微管蛋白(5 μM)及系列浓度的 Cabazitaxel(0.5 nM 至 20 nM)25°C 孵育 30 分钟。荧光偏振法检测竞争性结合,Cabazitaxel 与 β- 微管蛋白的解离常数(Kd)为 0.9 nM [1] |
| 细胞实验 |
抗增殖实验:PC3/DU145 细胞接种于 96 孔板(3×103 个细胞 / 孔),用系列浓度的游离 Cabazitaxel 或 Cabazitaxel 载药纳米粒(等效 0.01 nM 至 20 nM Cabazitaxel)处理 72 小时。MTT 法评估细胞活力,计算 IC50 值 [1][2]
- 细胞周期分析:PC3 细胞用 Cabazitaxel(0.5-2 nM)或纳米粒(等效 0.2-1 nM)处理 24 小时,70% 乙醇固定,碘化丙啶染色,流式细胞术定量 G2/M 期比例 [1] - 凋亡实验:DU145 细胞用 Cabazitaxel(1-2 nM)或骨靶向纳米粒(等效 0.5-1 nM)处理 48 小时,用膜联蛋白 V-FITC/碘化丙啶染色,流式细胞术分析。Western blot 检测半胱天冬酶 -3/PARP 切割 [2] - 克隆形成实验:PC3 细胞用 Cabazitaxel 或 Cabazitaxel-ICG 纳米粒(等效 0.5-1 nM)处理 24 小时后,接种于 6 孔板(1×103 个细胞 / 孔),孵育 14 天。菌落染色计数,相对于对照组计算抑制率 [1] |
| 动物实验 |
Murine tumor xenografts PC3 xenograft model: Female nude mice (6-8 weeks old) were subcutaneously implanted with 5×106 PC3 cells. When tumors reached 100-150 mm3, mice were randomized (n=8/group) and treated with: (1) vehicle (Cremophor EL + ethanol + saline) i.v., (2) free Cabazitaxel (10 mg/kg) i.v., q.o.d. for 21 days, (3) Cabazitaxel-ICG NPs (10 mg/kg Cabazitaxel equivalent) i.v., q.o.d. for 21 days. Tumor volume and weight were measured every 3 days [1] - Prostate cancer bone metastasis model: Male BALB/c nude mice (6-8 weeks old) were intra-tibially inoculated with 1×106 PC3-luc cells. Seven days later, mice were randomized (n=8/group) and treated with: (1) vehicle i.v., (2) free Cabazitaxel (8 mg/kg) i.v., weekly for 4 weeks, (3) bone-targeted Cabazitaxel NPs (8 mg/kg Cabazitaxel equivalent) i.v., weekly for 4 weeks. Bone lesions were monitored by bioluminescence imaging, and pain behavior was assessed by von Frey test [2] - Cabazitaxel NPs were formulated by encapsulating Cabazitaxel (and ICG for [1]) in biodegradable polymers, with particle size controlled at 100-150 nm [1][2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Based on the population pharmacokinetic analysis, after an intravenous dose of cabazitaxel 25 mg/m2 every three weeks, the mean Cmax in patients with metastatic prostate cancer was 226 ng/mL (CV 107%) and was reached at the end of the one-hour infusion (Tmax). The mean AUC in patients with metastatic prostate cancer was 991 ng x h/mL (CV 34%). No major deviation from the dose proportionality was observed from 10 to 30 mg/m2 in patients with advanced solid tumours. After a one-hour intravenous infusion [ 14 C]-cabazitaxel 25 mg/m 2 , approximately 80% of the administered dose was eliminated within two weeks. Cabazitaxel is mainly excreted in the feces as numerous metabolites (76% of the dose), while renal excretion of cabazitaxel and metabolites account for 3.7% of the dose (2.3% as unchanged drug in urine). Around 20 metabolites of cabazitaxel are excreted into human urine and feces. Steady-state volume of distribution (Vss) was 4,864 L (2,643 L/m 2 for a patient with a median BSA of 1.84 m 2 ). Based on the population pharmacokinetic analysis, cabazitaxel has a plasma clearance of 48.5 L/h (CV 39%; 26.4 L/h/m 2 for a patient with a median BSA of 1.84 m 2 ) in patients with metastatic prostate cancer. Metabolism / Metabolites More than 95% of cabazitaxel is extensively metabolized in the liver. CYP3A4 and CYP3A5 are responsible for 80% to 90% of drug metabolism, while CYP2C8 is involved to a lesser extent. While cabazitaxel is the main circulating moiety in human plasma, seven metabolites have been detected in plasma, including three active metabolites arising from O-demethylation - [docetaxel], RPR112698, and RPR123142. The main metabolite accounts for 5% of total cabazitaxel exposure. Biological Half-Life Following a one-hour intravenous infusion, plasma concentrations of cabazitaxel can be described by a three-compartment pharmacokinetic model with α-, β-, and γ- half-lives of four minutes, two hours, and 95 hours, respectively. In nude mice, Cabazitaxel-ICG NPs (10 mg/kg equivalent) showed a prolonged terminal half-life (t1/2 = 8.6 hours) vs free Cabazitaxel (t1/2 = 2.3 hours) [1] - Tumor uptake of Cabazitaxel-ICG NPs was 3.2-fold higher than free Cabazitaxel at 24 hours post-injection, with AUC0-24h in tumors increasing from 12.8 μM·h (free) to 41.5 μM·h (NPs) [1] - Bone-targeted Cabazitaxel NPs showed 4.5-fold higher bone tissue accumulation vs free Cabazitaxel, with reduced non-target organ (liver, kidney) uptake by 30-40% [2] - Human plasma protein binding of Cabazitaxel is 97% at therapeutic concentrations [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the clinical trials and open label studies of cabazitaxel in metastatic prostate cancer, serum enzyme elevations were usually not mentioned and hepatic adverse events did not appear in lists of serious adverse events. The product label for cabazitaxel states that elevations of serum ALT and AST above 5 times ULN occur in less than 1% of treated patients. Cabazitaxel has not been linked convincingly to instances of idiosyncratic, clinically apparent liver injury with jaundice. Cabazitaxel has been linked to acute hypersensitivity reactions that typically occur with the initial infusions and rarely with subsequent administration. Acute hypersensitivity reactions occur with the other taxanes (docetaxel and paclitaxel) which can be severe and lead to acute hepatic necrosis, multiorgan failure and death. While similar reactions have not been reported with cabazitaxel, its use has been limited. Thus, cabazitaxel has not been linked to instances of idiosyncratic, clinically apparent liver injury, but has been found to cause acute hypersensitivity reactions which have the potential to lead to acute hepatic necrosis (as have docetaxel and paclitaxel). Likelihood score: E (unproven, but suspected rare cause of clinically apparent liver injury). Protein Binding _In vitro_, the binding of cabazitaxel to human serum proteins was 89% to 92% and was not saturable up to 50,000 ng/mL. Cabazitaxel is mainly bound to human serum albumin (82%) and lipoproteins (88% for HDL, 70% for LDL, and 56% for VLDL). The _in vitro_ blood-to-plasma concentration ratio in human blood ranged from 0.90 to 0.99, indicating that cabazitaxel was equally distributed between blood and plasma. Free Cabazitaxel (10 mg/kg i.v.) caused mild myelosuppression (20% reduction in white blood cells) and transient liver enzyme elevation (1.5-fold) in mice, while Cabazitaxel-ICG NPs eliminated these toxicities [1] - Bone-targeted Cabazitaxel NPs (8 mg/kg i.v.) showed no significant histopathological abnormalities in liver, kidney, or bone marrow, compared to free Cabazitaxel (mild renal tubular injury) [2] - Mice treated with Cabazitaxel NPs showed no significant weight loss (<3%), while free Cabazitaxel caused 8% weight loss [1][2] |
| 参考文献 |
|
| 其他信息 |
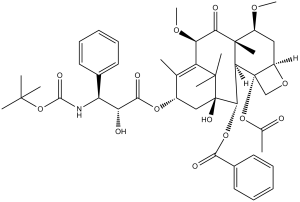
Cabazitaxel is a tetracyclic diterpenoid that is 10-deacetylbaccatin III having O-methyl groups attached at positions 7 and 10 as well as an O-(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl group attached at position 13. Acts as a microtubule inhibitor, binds tubulin and promotes microtubule assembly and simultaneously inhibits disassembly. It has a role as an antineoplastic agent and a microtubule-stabilising agent. It is functionally related to a 10-deacetylbaccatin III.
Cabazitaxel is a taxoid synthesized from 10-deacetylbaccatin III, a compound isolated from the yew tree. As a second-generation semisynthetic microtubule inhibitor, cabazitaxel stabilizes microtubules and induces tumour cell death. Due to its low affinity for the P-glycoprotein (P-gp) efflux pump, cabazitaxel can more readily penetrate the blood–brain barrier compared to other taxanes like [paclitaxel] and [docetaxel]. Cabazitaxel is used to treat metastatic castration-resistant prostate cancer. It was first approved by the FDA on June 17, 2010. It was also approved by the EMA on March 17, 2011 and Health Canada on December 17, 2019. Cabazitaxel is a Microtubule Inhibitor. The physiologic effect of cabazitaxel is by means of Microtubule Inhibition. Cabazitaxel is a taxane and antineoplastic agent which is currently used in the therapy of castration-resistant metastatic prostate cancer after failure of docetaxel. Therapy with cabazitaxel has been associated with a low rate of serum enzyme elevations, but has not been linked to cases of clinically apparent acute liver injury, although it can cause severe hypersensitivity infusion reactions which in some instances can be associated with acute liver injury. Cabazitaxel is a semi-synthetic derivative of the natural taxoid 10-deacetylbaccatin III with potential antineoplastic activity. Cabazitaxel binds to and stabilizes tubulin, resulting in the inhibition of microtubule depolymerization and cell division, cell cycle arrest in the G2/M phase, and the inhibition of tumor cell proliferation. Unlike other taxane compounds, this agent is a poor substrate for the membrane-associated, multidrug resistance (MDR), P-glycoprotein (P-gp) efflux pump and may be useful for treating multidrug-resistant tumors. In addition, cabazitaxel penetrates the blood-brain barrier (BBB). Drug Indication Cabazitaxel is indicated, in combination with [prednisone], for the treatment of patients with metastatic castration-resistant prostate cancer previously treated with a [docetaxel]-containing treatment regimen. In Europe and Canada, it can also be used in combination with [prednisolone]. Treatment of patients with hormone refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen. Jevtana in combination with prednisone or prednisolone is indicated for the treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen. Treatment of prostate cancer Mechanism of Action Microtubules are cytoskeletal polymers that regulate cell shape, vesicle transport, cell signalling, and cell division. They are made up of alpha-tubulin and beta-tubulin heterodimers. Microtubules extend toward the mitotic spindle during mitosis to allow the separation and distribution of chromosomes during cell division. Cabazitaxel binds to the N-terminal amino acids of the beta-tubulin subunit and promotes microtubule polymerization while simultaneously inhibiting disassembly: this results in the stabilization of microtubules, preventing microtubule cell division. Cabazitaxel ultimately blocks mitotic and interphase cellular functions and tumour proliferation. Pharmacodynamics Cabazitaxel demonstrates a broad spectrum of antitumour activity against advanced human tumours xenografted in mice, including intracranial human glioblastomas. Cabazitaxel has a low affinity to P-glycoprotein, allowing it to penetrate the blood-brain barrier without being subject to extensive P-gp-mediated active efflux. Cabazitaxel works against docetaxel-sensitive tumours and tumour models resistant to docetaxel and other chemotherapy drugs. Cabazitaxel is a semi-synthetic taxoid chemotherapy agent, structurally related to docetaxel but with improved activity against taxane-resistant prostate cancer [1][2] Its mechanism of action involves binding to β-tubulin, stabilizing microtubules, inhibiting microtubule depolymerization, inducing G2/M cell cycle arrest, and triggering caspase-dependent apoptosis [1][2] Nanoparticle delivery (tumor-targeted or bone-targeted) enhances Cabazitaxel’s solubility, tumor/bone tissue accumulation, and reduces systemic toxicity, improving therapeutic index [1][2] Cabazitaxel is clinically indicated for the treatment of metastatic castration-resistant prostate cancer (mCRPC), particularly in patients who have failed prior docetaxel therapy [1][2] Bone-targeted Cabazitaxel NPs not only inhibit bone metastatic lesions but also alleviate tumor-induced bone pain by reducing osteoclast activity and bone destruction [2] |
| 分子式 |
C45H57NO14
|
|---|---|
| 分子量 |
835.93
|
| 精确质量 |
835.377
|
| CAS号 |
183133-96-2
|
| 相关CAS号 |
Cabazitaxel-d6;1383561-29-2;Cabazitaxel-d9;1383572-19-7
|
| PubChem CID |
9854073
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
870.7±65.0 °C at 760 mmHg
|
| 熔点 |
180 °C
|
| 闪点 |
480.4±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.592
|
| LogP |
7.55
|
| tPSA |
202.45
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
15
|
| 重原子数目 |
60
|
| 分子复杂度/Complexity |
1690
|
| 定义原子立体中心数目 |
11
|
| SMILES |
CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)OC)C)OC
|
| InChi Key |
BMQGVNUXMIRLCK-OAGWZNDDSA-N
|
| InChi Code |
InChI=1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,32-,33+,34+,35-,37-,43+,44-,45+/m0/s1
|
| 化学名 |
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.
|
| 别名 |
TXD 258; XRP6258; RPR116258A; TXD-258; RPR-116258A; TXD258; XRP-6258; TXD 258; XRP 6258; RPR-116258A; trade name: Jevtana.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.99 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (2.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1963 mL | 5.9814 mL | 11.9627 mL | |
| 5 mM | 0.2393 mL | 1.1963 mL | 2.3925 mL | |
| 10 mM | 0.1196 mL | 0.5981 mL | 1.1963 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Cabazitaxel in Combination With 177Lu-PSMA-617 in Metastatic Castration-resistant Prostate Cancer
CTID: NCT05340374
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-11-08