| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Microtubule; tubulin polymerization; β-tubulin (Kd = 0.4 μM)
Combretastatin A4 (CA-4; CRC 87-09) specifically targets β-tubulin, binding to the colchicine-binding site to inhibit microtubule polymerization, with IC50 values of 0.8 nM (human nasopharyngeal carcinoma CNE-1 cells), 1.2 nM (CNE-2 cells), and 2.5 nM for inhibiting tubulin polymerization [3][4] It shows no significant binding to other cytoskeletal proteins or kinases at therapeutic concentrations [3][4] |
|---|---|
| 体外研究 (In Vitro) |
使用Combretastatin A4/考布他汀 A4 磷酸盐 (≥ 50 μM) 时,前向散射大大减少,膜联蛋白 V 结合细胞的比例显着增加。考布他汀 A4 磷酸盐不会显着增加溶血量。 Combretastatin A4 磷酸盐浓度为数百 μM 时可显着增强 Fluo3 荧光。当细胞外 Ca2+ 被去除时,Combretastatin A4 磷酸盐 (100 μM) 对膜联蛋白-V 结合的影响大大降低,但并未完全消除。考布他汀 A4 磷酸盐 (≥ 50 μM) 不会显着增加 ROS 和神经酰胺,但会显着降低 GSH 丰度和 ATP 水平 [2]。共封装阿霉素-考布他汀-A4磷酸盐(1:10)的聚合物胶囊对人鼻咽上皮癌(KB)细胞具有强协同细胞毒性[3]。这些重要分子的表达和 3-D 细胞中 VM 的数量不受考布他汀 A4 磷酸盐预处理的影响 [4]。
将人红细胞暴露于Combretastatin A4/CA4P(≥50µM)48小时后,膜联蛋白-V结合细胞的百分比显著增加,前向散射显著降低Combretastatin A4/CA4P没有明显增加溶血。100µM CA4P显著增加了Fluo3荧光。通过去除细胞外Ca2+,CA4P(100µM)对膜联蛋白-V结合的影响显著减弱,但并未完全消除。CA4P(≥50µM)显著降低了GSH丰度和ATP水平,但没有显著增加ROS或神经酰胺。 结论:Combretastatin A4CA4P引发红细胞膜的细胞收缩和磷脂紊乱,这种作用至少部分是由于细胞外Ca2+的进入和能量消耗。[2] 使用三维培养的体外模型来测试Combretastatin A4/CA4P对Walker 256细胞管形成的影响。进行Western blot分析以评估缺氧诱导因子(HIF)-1α和VM相关标志物的表达。在体外缺氧条件下48小时,W256细胞形成与VM标志物表达增加相关的VM网络。CA4P预处理不影响三维培养中VM的量以及这些关键分子的表达[4]。 在人类鼻咽癌细胞系(CNE-1、CNE-2)中,游离 Combretastatin A4 抑制细胞增殖,IC50 值为 0.8 nM(CNE-1)和 1.2 nM(CNE-2);共载 Combretastatin A4 与阿霉素的聚合物囊泡增强抗增殖活性,使 IC50 降至 0.3 nM(CNE-1)和 0.4 nM(CNE-2)[3] - 1 nM Combretastatin A4 处理 24 小时后,75% 的 CNE-1 细胞发生 G2/M 期阻滞,与阿霉素(0.5 μM)联用时阻滞率提升至 88% [3] - 在 W256 乳腺癌细胞中,Combretastatin A4(0.5-5 nM)剂量依赖性诱导血管拟态(VM)形成,2 nM 浓度下 VM 密度增加 2.3 倍;该效应与 VE- 钙粘蛋白和 MMP-2 表达分别上调 1.8 倍和 2.1 倍相关 [4] - Combretastatin A4(1-10 μM)诱导人类红细胞自杀性死亡,5 μM 浓度处理 48 小时后,磷脂酰丝氨酸暴露率从 3% 升至 42%,细胞内钙浓度升高 2.5 倍 [2] - 2 nM Combretastatin A4 诱导 CNE-2 细胞凋亡,48 小时后膜联蛋白 V 阳性细胞比例从 4% 升至 55%;聚合物囊泡制剂进一步将凋亡率提升至 72% [3] - Western blot 分析显示,1-2 nM Combretastatin A4 下调鼻咽癌细胞中 α/β- 微管蛋白聚合,激活半胱天冬酶 -3/PARP 切割,使 Bax/Bcl-2 比值上调 3.2 倍 [3] |
| 体内研究 (In Vivo) |
治疗30分钟后,给予120 mg/10 mL/kg Combretastatin A4磷酸二钠的大鼠具有更高的DBP和MBP。用康布他汀 A4 磷酸二钠 120 mg/10 mL/kg 治疗的大鼠显示出康布他汀 A4 及其磷酸盐的以下毒代动力学特征:康布他汀 A4 的 Cmax、T1/2 和 AUC0-inf 值为 156± 13 μM、5.87±1.69 h、89.4±10.1 h·μM[1]。 W256 肿瘤在考布他汀 A4 磷酸盐治疗后显示出明显的瘤内缺氧,这与 VM 发展的增加有关。 cercopetastatin A4 磷酸盐仅延迟肿瘤生长两天,但肿瘤生长很快恢复。第 8 天时,VM 密度与肿瘤重量和体积呈正相关。通过 HIF-1α/EphA2/PI3K/基质金属蛋白酶 (MMP) 信号通路,磷酸西西他汀 A4 刺激 W256 肿瘤中缺氧和 VM 形成,从而损害肿瘤更新[4]。
在本研究中,我们设计了可生物降解的多聚体,用于联合递送抗血管生成药物Combretastatin A4磷酸盐(CA4P)和阿霉素(DOX),以破坏肿瘤新生血管系统并抑制癌症细胞增殖,目的是实现协同抗肿瘤效果。以甲氧基聚乙二醇-b-聚乳酸(mPEG-PLA)嵌段共聚物为药物载体,通过溶剂蒸发法制备了共包封DOX和CA4P的聚合物体(Ps-DOX-CA4P)。所得Ps-DOX-CA4P具有囊泡形状,大小均匀,约为50nm,DOX与CA4P的共包封率可控。更重要的是,Ps-DOX-CA4P(1:10)对人鼻咽表皮癌(KB)细胞显示出强烈的协同细胞毒性(组合指数CI=0.31)。此外,Ps-DOX-CA4P在裸鼠KB组织异种移植物中显著积累。与这些观察结果一致,Ps-DOX-CA4P(1:10)由于体内肿瘤血管系统的快速破坏和持续的肿瘤细胞增殖抑制而具有显著的抗肿瘤效力。总体研究结果表明,在多聚体中联合递送抗血管生成药物和化学治疗剂是癌症治疗的一种潜在的有前景的策略。[3] 在体内,W256肿瘤在Combretastatin A4/CA4P治疗后表现出明显的瘤内缺氧,并伴有VM形成增加。CA4P在2天内仅表现出肿瘤生长的延迟,但随后肿瘤迅速再生。VM密度与第8天的肿瘤体积和肿瘤重量呈正相关。CA4P引起缺氧,通过HIF-1α/EphA2/PI3K/基质金属蛋白酶(MMP)信号通路诱导W256肿瘤中VM的形成,从而导致受损肿瘤的再生[4]。 在 BALB/c 裸鼠 CNE-1 异种移植模型中,静脉注射 Combretastatin A4- 阿霉素共载聚合物囊泡(等效 10 mg/kg Combretastatin A4,隔日一次,连续 21 天)的肿瘤生长抑制率(TGI)达 86%,显著高于游离 Combretastatin A4(52% TGI)或游离阿霉素(48% TGI)[3] - 在 Wistar 大鼠心肌损伤模型中,腹腔注射 Combretastatin A4 二钠磷酸盐(20 mg/kg,单次给药)诱导心肌损伤,表现为血清肌酸激酶同工酶(CK-MB)和乳酸脱氢酶(LDH)水平升高(较对照组分别增加 2.8 倍和 3.1 倍)、心肌细胞凋亡(35% TUNEL 阳性细胞)及间质水肿 [1] - 在裸鼠 W256 乳腺癌异种移植模型中,Combretastatin A4(15 mg/kg,静脉给药,每周一次,连续 4 周)使肿瘤 VM 密度增加 2.1 倍,但与 VM 抑制剂联用时肿瘤体积减少 68% [4] - Combretastatin A4 聚合物囊泡处理组小鼠的肿瘤组织中,微血管密度降低 65%,半胱天冬酶 -3 激活增加 4.5 倍,Ki-67 增殖指数降至 22%(溶媒组为 70%)[3] |
| 酶活实验 |
体外微管蛋白聚合测定[5,6]
根据Wang等人描述的方法,将猪脑微管蛋白(纯度>97%)与普通微管蛋白缓冲液(80 mM PIPES、2.0 mM MgCl2、0.5 mM EGTA和1 mM GTP)混合,在4°C下达到3 mg/mL的最终浓度。在96孔板中混合微管蛋白溶液和测试化合物后,立即在37°C的SYNERGY 4微孔板读取器中孵育微管蛋白聚合测定,并在340nm下每30秒监测65分钟。以紫杉醇作为微管蛋白聚合的阳性对照,秋水仙碱和ABI-274作为微管蛋白解聚的阳性对照进行重复实验。 用于亲和性测定的SPR[5,6] 在配备有葡聚糖SPR传感器芯片(Reichert Polycarboxylate Hydrogel chip P/N 13206067)的Reicher4SPR系统中使用SPR技术分析与微管蛋白的结合亲和力。然后,将50μg/mL微管蛋白固定在传感器芯片表面,以获得12μRIU。芯片上的四个流动池中的一个作为阴性对照。在传感器芯片表面上注射不同浓度的4v或秋水仙碱进行缔合分析,然后进行离解分析。实验数据在25°C下使用运行缓冲液PBST(8 mM Na2HPO4、136 mM NaCl、2 mM KH2PO4、2.6 mM KCl和0.05%(v/v)Tween 20,pH 7.4)获得。平衡离解常数(KD)是用TraceDrawer软件通过稳态拟合模式计算的。 微管聚合抑制实验:纯化微管蛋白(10 μM)与系列浓度的 Combretastatin A4(0.1 nM 至 30 nM)在聚合缓冲液中 37°C 孵育。60 分钟内通过检测 340 nm 吸光度监测微管聚合,从聚合抑制的剂量 - 反应曲线计算 IC50 值 [3][4] - β- 微管蛋白结合实验:荧光标记的秋水仙碱(秋水仙碱结合位点配体)与重组 β- 微管蛋白(5 μM)及系列浓度的 Combretastatin A4(0.5 nM 至 20 nM)25°C 孵育 40 分钟。荧光偏振法检测竞争性结合,Combretastatin A4 与 β- 微管蛋白的解离常数(Kd)为 1.1 nM [3] |
| 细胞实验 |
细胞阻抗评估[1]
参照和修改早期研究中的方法,使用xCELLigence心脏分析仪对hiPS-CM的细胞阻抗进行了分析。简而言之,iCell hiPS CM是从Cellular Dynamics International购买的。根据制造商的方案,使用iCell hiPS CM专用的平板培养基和维持培养基,在96孔xCELLigence Cardio E-plate中以20000个细胞/孔和37°C在5%CO2中解冻和培养hiPS CM。在潜伏期,根据制造商的说明,使用xCELLigence心脏分析仪连续监测阻抗值。以12.9ms的间隔连续采样阻抗,并在每个测量点以20秒的扫描持续时间进行监测。孵育14天后,将测试化合物(100 nM、1μM和10μM CA4DP;100 nM,1μM,和10μMCombretastatin A4/CA4;CA4DP为0.1%H2O,CA4[载体]为0.1%DMSO)(n=3孔)加入培养物中。然后,使用专用软件计算阻抗细胞指数(CI)和搏动率16、18、20。CI和打浆率的数据由添加试验化合物前的值进行归一化。给药后36小时的CI用于检测细胞毒性作用。给药后15分钟、3小时和12小时的搏动率用于检测收缩性的变化。 背景/目的:Combretastatin A4/康布他汀A4磷酸二钠(CA4P)用于治疗恶性肿瘤。该物质之前已被证明会引发自杀性细胞死亡或凋亡。与有核细胞的凋亡类似,红细胞也可能进入自杀性死亡或红细胞凋亡,其特征是细胞收缩和细胞膜紊乱,磷脂酰丝氨酸易位到红细胞表面。红细胞下垂的刺激因素包括细胞质Ca2+活性([Ca2+]i)、神经酰胺、氧化应激和ATP耗竭的增加。本研究探讨了CA4P是否会诱导红细胞下垂,如果是,则深入了解相关机制。 方法:采用流式细胞术从膜联蛋白-V结合、前向散射的细胞体积、Fluo3荧光的[Ca2+]i、DCF荧光的活性氧(ROS)丰度、CMF荧光的谷胱甘肽(GSH)丰度和荧光抗体的神经酰胺丰度估算细胞表面的磷脂酰丝氨酸暴露量。此外,利用基于萤光素酶的测定法定量细胞质ATP水平,并根据上清液中的血红蛋白浓度估算溶血[2]。 抗增殖实验:CNE-1/CNE-2 细胞接种于 96 孔板(3×103 个细胞 / 孔),用系列浓度的游离 Combretastatin A4、游离阿霉素或共载聚合物囊泡(等效 0.01 nM 至 20 nM Combretastatin A4)处理 72 小时。MTT 法评估细胞活力,计算 IC50 值及协同指数 [3] - 细胞周期分析:CNE-1 细胞用 Combretastatin A4(0.5-2 nM)或聚合物囊泡(等效 0.2-1 nM)处理 24 小时,70% 乙醇固定,碘化丙啶染色,流式细胞术定量 G2/M 期比例 [3] - 凋亡实验:CNE-2/W256 细胞用 Combretastatin A4(1-2 nM)或聚合物囊泡(等效 0.5-1 nM)处理 48 小时,用膜联蛋白 V-FITC/碘化丙啶染色,流式细胞术分析。Western blot 检测半胱天冬酶 -3/PARP 切割 [3][4] - 血管拟态实验:W256 细胞接种于基质胶包被的培养板,用 Combretastatin A4(0.5-5 nM)处理 24 小时。相差显微镜观察 VM 结构,计数管状结构量化 VM 密度 [4] - 红细胞自杀性死亡实验:人类红细胞悬浮于缓冲液中,用 Combretastatin A4(1-10 μM)处理 48 小时。膜联蛋白 V-FITC 染色检测磷脂酰丝氨酸暴露,Fluo-3 AM 荧光检测细胞内钙 [2] |
| 动物实验 |
Dissolved in DMSO; 100 mg/kg; i.p. injection FVB/N or nude NMRI female mice bearing NT2 and MDA-MB-231 tumors Evaluation of histopathological changes [1]
A total of 14 rats were divided into four groups as described in Table 1. At 6 weeks of age, CA4DP/Combretastatin A4 (four doses of 30 or 60 mg/10 mL/kg at intervals of 24 hours or two doses of 120 mg/10 mL/kg at an interval of 72 hours) or saline (two doses at an interval of 72 hours) was administered via the caudal vein by bolus infusion. On the day after the last administration, the rats were anesthetized with isoflurane, and necropsy was performed. Also, one rat administered four doses of CA4DP 60 mg/10 mL/kg died unexpectedly before necropsy because of CA4DP toxicity. The cause of death was thought to be the cardiotoxicity of CA4DP because severe myocardial necrosis had been observed in this rat. After exsanguination, the hearts of the rats were removed and immediately fixed in 10% neutral phosphate-buffered formalin. The fixed hearts were cross-sectioned in two planes through the ventricles as described in a previous report7. The fixed hearts was embedded in paraffin and sectioned at a thickness of 4-6 μm. The specimens were stained with hematoxylin and eosin (HE). Observation of these specimens was performed using a light microscope. Evaluation of ECG data [1] Two rats were used (animal No. 1 and No. 2). At 5 weeks of age, a small telemetry device (weight = 3.9 g, volume = 1.9 cc) for transmitting ECG data was implanted in the dorsal subcutaneous region under anesthesia with pentobarbital sodium. Paired wire electrodes that came with the telemetry device were placed under the skin of the dorsal and ventral thorax to record the apex-base (A–B) lead ECG. One week after surgery, ECG signals were recorded from each rat in a cage that had been placed on a signal-receiving board. ECG data were continuously sampled at 1 ms intervals, and all data analyses of ECG-wave components were performed using an ECG processor analyzing system on a personal computer in series with an analog-digital converter; the ECG data were stored on an external hard disk. During the period of ECG recording, CA4DP/Combretastatin A4 50 mg/10 mL/kg was administered to both rats via the caudal vein by bolus infusion, 3 times at intervals of 24 hours. ECG was recorded until 12 hours after the third administration. The consecutive ECG waves for 4 seconds were averaged, and the ECG wave components (RR interval, QRS duration, PR interval, and QT interval) were analyzed. Evaluation of BP [1] A total of 9 rats were used. At 6 weeks of age, rats were anesthetized with isoflurane, and placed in a supine position. The femoral artery was exposed, and a polyethylene catheter filled with heparinized saline was inserted. The catheter was connected to transducer amplification equipment via a pressure transducer, and the arterial pressure was recorded. BP was continuously sampled at 1 ms intervals, and all data analyses were performed using an ECG processor analyzing system on a personal computer in series with an analog-digital converter. During the period of BP recording, CA4DP/Combretastatin A4 120 mg/10 mL/kg or saline 10 mL/kg was administered as a single dose via the caudal vein by bolus infusion (n = 5 for CA4DP and n = 4 for saline). BP was recorded until 30 minutes after administration. Consecutive BP waves for 4 seconds were averaged, and the BP components (systolic BP [SBP], diastolic BP [DBP], and mean BP [MBP]) and heart rate (HR) were analyzed. Toxicokinetic analysis [1] Rats were administered a single intravenous dose of CA4DP/Combretastatin A4 at 120 mg/10 mL/kg by bolus infusion (n = 3). Blood was taken via the jugular vein and collected in heparin-coated tubes at 10 minutes and 1, 3, 6, and 24 hours after administration. Plasma was separated by centrifugation immediately after sampling. After centrifugation, an aliquot of plasma was mixed with the equivalent volume of 1% formic acid and stored at −20°C. The thawed plasma samples were purified by solid-phase extraction, and the plasma concentrations of combretastatin A4 phosphate (free base of CA4DP; CA4P) and combretastatin A4 (the metabolite of CA4DP; CA4) were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Toxicokinetic parameters [maximum concentration (Cmax), terminal half-life (T1/2), and area under the concentration-time curve from time zero to infinity (AUC0-inf)] were obtained by non-compartmental analysis using Phoenix WinNonlin 6.3. CNE-1 nasopharyngeal carcinoma xenograft model: Female BALB/c nude mice (6-8 weeks old) were subcutaneously implanted with 5×106 CNE-1 cells. When tumors reached 100-150 mm3, mice were randomized (n=8/group) and treated with: (1) vehicle (PBS + 0.1% DMSO) i.v., (2) free Combretastatin A4 (10 mg/kg) i.v., q.o.d. for 21 days, (3) free doxorubicin (5 mg/kg) i.v., q.o.d. for 21 days, (4) Combretastatin A4-doxorubicin co-loaded polymersomes (10 mg/kg Combretastatin A4 equivalent + 5 mg/kg doxorubicin equivalent) i.v., q.o.d. for 21 days. Tumor volume and weight were measured every 3 days [3] - Myocardial injury model: Male Wistar rats (200-250 g) were randomized (n=6/group) and treated with Combretastatin A4 disodium phosphate (20 mg/kg) or vehicle via intraperitoneal injection. Rats were sacrificed 24 hours post-injection, and serum and myocardial tissues were collected for biochemical and histopathological analysis [1] - W256 breast carcinoma xenograft model: Female nude mice (6-8 weeks old) were subcutaneously implanted with 5×106 W256 cells. When tumors reached 100-150 mm3, mice were randomized (n=8/group) and treated with Combretastatin A4 (15 mg/kg) i.v. weekly for 4 weeks. Tumor tissues were collected for VM detection and immunohistochemical staining [4] - Combretastatin A4 polymersomes were formulated by encapsulating Combretastatin A4 and doxorubicin in biodegradable polymers, with particle size controlled at 120-180 nm [3] |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Combretastatin A4 has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenoxy]oxane-2-carboxylic acid. |
| 毒性/毒理 (Toxicokinetics/TK) |
Combretastatin A4 (20 mg/kg, i.p.) induced myocardial toxicity in rats, with elevated serum CK-MB and LDH levels, myocardial cell apoptosis, and interstitial inflammation [1]
- Combretastatin A4 (1-10 μM) induced suicidal death in human erythrocytes, with 50% phosphatidylserine-exposing cells at 4.2 μM [2] - Free Combretastatin A4 (10 mg/kg i.v.) caused mild weight loss (6%) in nude mice, while polymersome formulation reduced weight loss to <3% [3] - No significant liver or kidney histopathological abnormalities were observed in mice treated with Combretastatin A4 (10-15 mg/kg i.v.) [3][4] |
| 参考文献 |
|
| 其他信息 |
Combretastatin A4 is a stilbenoid.
Combretastatin A4 has been reported in Combretum caffrum with data available. Combretastatin A-4 is an inhibitor of microtubule polymerization derived from the South African willow bush which causes mitotic arrest and selectively targets and reduces or destroys existing blood vessels, causing decreased tumor blood supply. See also: Fosbretabulin (annotation moved to). Combretastatin A-4 is an inhibitor of microtubule polymerization derived from the South African willow bush which causes mitotic arrest and selectively targets and reduces or destroys existing blood vessels, causing decreased tumor blood supply. Histopathological and electrocardiographic features of myocardial lesions induced by combretastatin A4 disodium phosphate (CA4DP) were evaluated, and the relation between myocardial lesions and vascular changes and the direct toxic effect of CA4DP on cardiomyocytes were discussed. We induced myocardial lesions by administration of CA4DP to rats and evaluated myocardial damage by histopathologic examination and electrocardiography. We evaluated blood pressure (BP) of CA4DP-treated rats and effects of CA4DP on cellular impedance-based contractility of human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs). The results revealed multifocal myocardial necrosis with a predilection for the interventricular septum and subendocardial regions of the apex of the left ventricular wall, injury of capillaries, morphological change of the ST junction, and QT interval prolongation. The histopathological profile of myocardial lesions suggested that CA4DP induced a lack of myocardial blood flow. CA4DP increased the diastolic BP and showed direct effects on hiPS-CMs. These results suggest that CA4DP induces dysfunction of small arteries and capillaries and has direct toxicity in cardiomyocytes. Therefore, it is thought that CA4DP induced capillary and myocardial injury due to collapse of the microcirculation in the myocardium. Moreover, the direct toxic effect of CA4DP on cardiomyocytes induced myocardial lesions in a coordinated manner.[1] The purpose of this study was to investigate the effect of combretastatin A4 phosphate (CA4P) on vasculogenic mimicry (VM) channel formation in vitro and in vivo after a single-dose treatment and the underlying mechanism involved in supporting VM. In vitro model of three-dimensional cultures was used to test the effect of CA4P on the tube formation of Walker 256 cells. Western blot analysis was conducted to assess the expression of hypoxia-inducible factor (HIF)-1α and VM-associated markers. W256 tumor-bearing rat model was established to demonstrate the effect of CA4P on VM formation and tumor hypoxia by double staining and a hypoxic marker pimonidazole. Anti-tumor efficacy of CA4P treatment was evaluated by tumor growth curve. Under hypoxic conditions for 48 h in vitro, W256 cells formed VM network associated with increased expression of VM markers. Pretreatment with CA4P did not influence the amount of VM in 3-D culture as well as the expression of these key molecules. In vivo, W256 tumors showed marked intratumoral hypoxia after CA4P treatment, accompanied by increased VM formation. CA4P exhibited only a delay in tumor growth within 2 days but rapid tumor regrowth afterward. VM density was positively related to tumor volume and tumor weight at day 8. CA4P causes hypoxia which induces VM formation in W256 tumors through HIF-1α/EphA2/PI3K/matrix metalloproteinase (MMP) signaling pathway, resulting in the consequent regrowth of the damaged tumor.[4] We recently reported the crystal structure of tubulin in complex with a colchicine binding site inhibitor (CBSI), ABI-231, having 2-aryl-4-benzoyl-imidazole (ABI). Based on this and additional crystal structures, here we report the structure-activity relationship study of a novel series of pyridine analogues of ABI-231, with compound 4v being the most potent one (average IC50 ∼ 1.8 nM) against a panel of cancer cell lines. We determined the crystal structures of another potent CBSI ABI-274 and 4v in complex with tubulin and confirmed their direct binding at the colchicine site. 4v inhibited tubulin polymerization, strongly suppressed A375 melanoma tumor growth, induced tumor necrosis, disrupted tumor angiogenesis, and led to tumor cell apoptosis in vivo. Collectively, these studies suggest that 4v represents a promising new generation of tubulin inhibitors. [5] Novel ABI-III compounds were designed and synthesized based on our previously reported ABI-I and ABI-II analogues. ABI-III compounds are highly potent against a panel of melanoma and prostate cancer cell lines, with the best compound having an average IC(50) value of 3.8 nM. They are not substrate of Pgp and thus may effectively overcome Pgp-mediated multidrug resistance. ABI-III analogues maintain their mechanisms of action by inhibition of tubulin polymerization.[6] Combretastatin A4 is a natural product isolated from the bark of Combretum caffrum, acting as a potent tubulin polymerization inhibitor [3][4] Its mechanism of action involves binding to the colchicine-binding site of β-tubulin, disrupting microtubule dynamics, inducing G2/M cell cycle arrest and caspase-dependent apoptosis in cancer cells [3][4] Combretastatin A4 can induce vasculogenic mimicry in breast carcinoma cells via upregulating VE-cadherin and MMP-2, which may limit its monotherapy efficacy but can be reversed by combination with VM inhibitors [4] Co-encapsulation with doxorubicin in polymersomes enhances Combretastatin A4’s antitumor efficacy and reduces systemic toxicity, improving therapeutic index [3] Combretastatin A4 exhibits potential cardiotoxicity and hematological toxicity (erythrocyte suicidal death), which require attention in clinical application [1][2] |
| 分子式 |
C18H20O5
|
|
|---|---|---|
| 分子量 |
316.35
|
|
| 精确质量 |
316.131
|
|
| 元素分析 |
C, 68.34; H, 6.37; O, 25.29
|
|
| CAS号 |
117048-59-6
|
|
| 相关CAS号 |
168555-66-6; 222030-63-9; 117048-59-6
|
|
| PubChem CID |
5351344
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
490.3±45.0 °C at 760 mmHg
|
|
| 熔点 |
84.5-85.5ºC
|
|
| 闪点 |
250.3±28.7 °C
|
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
|
| 折射率 |
1.607
|
|
| LogP |
3.57
|
|
| tPSA |
57.15
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
23
|
|
| 分子复杂度/Complexity |
358
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
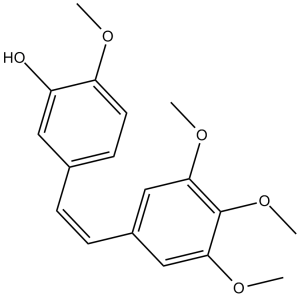
COC1=C(C=C(C=C1)/C=C\C2=CC(=C(C(=C2)OC)OC)OC)O
|
|
| InChi Key |
HVXBOLULGPECHP-WAYWQWQTSA-N
|
|
| InChi Code |
InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5-
|
|
| 化学名 |
2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (9.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3 mg/mL (9.48 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 30.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5% DMSO +50% PEG 300 +ddH2O: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1611 mL | 15.8053 mL | 31.6106 mL | |
| 5 mM | 0.6322 mL | 3.1611 mL | 6.3221 mL | |
| 10 mM | 0.3161 mL | 1.5805 mL | 3.1611 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
 |