| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
VEGFR2 (IC50 = 0.035 nM); Flt-4 (IC50 = 6 nM); Flt-1 (IC50 = 12 nM); Met (IC50 = 1.3 ± 1.2 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:XL184对RON和PDGFRβ具有弱抑制活性,IC50分别为124 nM和234 nM,对FGFR1具有低活性,IC50为5.294 μM。低浓度(0.1-0.5 μM)的 XL184 足以显着抑制 MPNST 细胞中的组成型和诱导型 Met 磷酸化及其由此产生的下游信号传导,并抑制 HGF 诱导的 MPNST 细胞迁移和侵袭。 XL184 还可显着抑制细胞因子刺激的人脐静脉内皮细胞 (HUVEC) 中的 Met 和 VEGFR2 磷酸化。虽然 0.1 μM 的 XL-184 对 MPNST 细胞的生长没有显着影响,但 5-10 μM 的 XL184 显着抑制 MPNST 细胞的生长。激酶测定:Cabozantinib (XL184, BMS-907351) 是一种有效的 VEGFR2 抑制剂,IC50 为 0.035 nM,还抑制 c-Met、Ret、Kit、Flt-1/3/4、Tie2 和 AXL,IC50 为 1.3 nM,分别为 4 nM、4.6 nM、12 nM/11.3 nM/6 nM、14.3 nM 和 7 nM。细胞测定:将细胞(ST88-14、STS26T 和 MPNST724)暴露于不同浓度的 XL184 中 48 小时。使用 CellTiter96 水性非放射性细胞增殖测定试剂盒通过 MTS 测定测定细胞生长。在 490 nm 波长处测量吸光度,处理细胞的吸光度值以未处理细胞吸光度的百分比表示。
|
| 体内研究 (In Vivo) |
对患有自发性胰岛肿瘤的 RIP-Tag2 小鼠进行 30 mg/kg XL184 治疗会破坏 83% 的肿瘤脉管系统,减少周细胞和空基膜套,导致广泛的瘤内缺氧和广泛的肿瘤细胞凋亡,并减缓肿瘤脉管系统的再生停药后,与阻断 VEGFR 但不阻断 c-Met 的 XL999 相比,效果更显着,导致血管分布仅减少 43%,表明同时抑制 VEGFR 和其他功能相关受体酪氨酸激酶 (RTK) 会放大血管生成抑制。 XL184 还可以降低原发肿瘤的侵袭性并减少转移。 30 mg/kg/天的 XL184 可显着消除 SCID 小鼠中的人类 MPNST 异种移植物的生长和转移。 XL184 的给药可诱导乳腺、肺和神经胶质瘤模型中肿瘤生长的剂量依赖性抑制,与肿瘤和内皮细胞增殖减少以及细胞凋亡增加相关。单次口服剂量的 XL184 足以分别以 100 mg/kg 和 10 mg/kg 剂量诱导 MDA-MB-231 荷瘤小鼠和 C6 荷瘤大鼠持续抑制肿瘤生长。
|
| 酶活实验 |
除了抑制 c-Met、Ret、Kit、Flt-1/3/4、Tie2 和 AXL 外,IC50 值为 1.3 nM、4 nM、4.6 nM、12 nM/11.3 nM/6 nM、14.3 nM 和分别为 7 nM 的卡博替尼 (XL184、BMS-907351) 是一种有效的 VEGFR2 抑制剂。
|
| 细胞实验 |
48 小时内,对细胞施加不同浓度的 XL184。使用 CellTiter96 水性非放射性细胞增殖检测试剂盒,MTS 检测可用于测量细胞生长。测量吸光度的波长为 490 nm,处理细胞的吸光度值表示为未处理细胞吸光度的百分比。
|
| 动物实验 |
RIP-Tag2 transgenic mice in a C57BL/6 background with spontaneous pancreatic islet tumors
~60 mg/kg Oral gavage |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After oral administration, peak plasma concentration was achieved in 2-5 hours. Cabozantinib is eliminated mostly by the feces (54%) and also by the urine (27%). The volume of distribution is 349L. At steady state, the clearance is 4.4 L/hr. Metabolism / Metabolites Cabozantinib is metabolized mostly by CYP3A4 and, to a minor extent, by CYP2C9. Both enzyme produce an N-oxide metabolite. Biological Half-Life Cabozantinib has a long half-life of 55 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of cabozantinib, elevations in serum aminotransferase levels were common, occurring in 16% to 97% of patients. Values greater than 5 times the upper limit of normal (ULN), however, occurred in only 2% to 8% of recipients. Serum alkaline phosphatase elevations were also common and were above 3 times ULN in 3% of patients. Despite the high rate of serum enzyme elevations, cases of clinically apparent liver injury including acute liver failure were not reported in the preregistration trials of cabozantinib. Since the approval of cabozantinib, there have been no published case reports of hepatotoxicity attributed to its use. Serum ALT, AST and alkaline phosphatase elevations are listed as adverse reactions in the product label for cabozantinib, and cholestatic hepatitis is mentioned as a rare occurrence, but monitoring of serum enzymes during treatment is not specifically recommended. Likelihood score: E* (Unproven but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of cabozantinib during breastfeeding. Because cabozantinib is more than 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life ranges from 55 to 99 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during cabozantinib therapy and for 4 months after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Cabozantinib has extensive plasma protein binding (≥ 99.7%). |
| 参考文献 | |
| 其他信息 |
Cabozantinib is a dicarboxylic acid diamide that is N-phenyl-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide in which the hydrogen at position 4 on the phenyl ring is substituted by a (6,7-dimethoxyquinolin-4-yl)oxy group. A multi-tyrosine kinase inhibitor, used (as its malate salt) for the treatment of progressive, metastatic, medullary thyroid cancer. It has a role as a tyrosine kinase inhibitor and an antineoplastic agent. It is a member of quinolines, an organofluorine compound, an aromatic ether and a dicarboxylic acid diamide.
Cabozantinib was first approved in 2012 and is a non-specific tyrosine kinase inhibitor. It was initially approved in the US under the brand name Cometriq, which is indicated for the treatment of metastatic medullary thyroid cancer. In 2016, a capsule formulation (Cabometyx) was approved for the treatment of advanced renal cell carcinoma, and this same formulation gained additional approval in both the US and Canada in 2019 for the treatment of hepatocellular carcinoma in previously treated patients. Cabozantinib is a Kinase Inhibitor. The mechanism of action of cabozantinib is as a Protein Kinase Inhibitor. Cabozantinib is orally available kinase inhibitor and antineoplastic agent that is used in treatment of advanced, metastatic medullary thyroid cancer and refractory renal cell carcinoma. Cabozantinib is associated with a low rate of serum enzyme elevations during treatment and has been implicated with rare instances of clinically apparent, acute liver injury, some of which have been severe. Cabozantinib is an orally bioavailable, small molecule receptor tyrosine kinase (RTK) inhibitor with potential antineoplastic activity. Cabozantinib strongly binds to and inhibits several RTKs, which are often overexpressed in a variety of cancer cell types, including hepatocyte growth factor receptor (MET), RET (rearranged during transfection), vascular endothelial growth factor receptor types 1 (VEGFR-1), 2 (VEGFR-2), and 3 (VEGFR-3), mast/stem cell growth factor (KIT), FMS-like tyrosine kinase 3 (FLT-3), TIE-2 (TEK tyrosine kinase, endothelial), tropomyosin-related kinase B (TRKB) and AXL. This may result in an inhibition of both tumor growth and angiogenesis, and eventually lead to tumor regression. See also: Cabozantinib s-malate (has salt form). Drug Indication Cabozantinib is indicated for the treatment of progressive, metastatic medullary thyroid cancer. It is also indicated for the treatment of advanced renal cell carcinoma and for hepatocellular carcinoma in patients previously treated with sorafenib. FDA Label Treatment of adult patients with progressive, unresectable locally advanced or metastatic medullary thyroid carcinoma. Renal Cell Carcinoma (RCC)Cabometyx is indicated as monotherapy for the treatment of advanced renal cell carcinoma (RCC): in treatment-naïve adults with intermediate or poor risk,in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy. Cabometyx, in combination with nivolumab, is indicated for the first-line treatment of advanced renal cell carcinoma in adults. Hepatocellular Carcinoma (HCC)Cabometyx is indicated as monotherapy for the treatment of hepatocellular carcinoma (HCC) in adults who have previously been treated with sorafenib. Treatment of solid malignant tumours Mechanism of Action Cabozantinib inhibits specific receptor tyrosine kinases such as VEGFR-1, -2 and -3, KIT, TRKB, FLT-3, AXL, RET, MET, and TIE-2. Pharmacodynamics Cabozantinib suppresses metastasis, angiogenesis, and oncognesis by inhibiting receptor tyrosine kinases. |
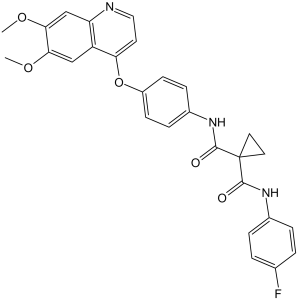
| 分子式 |
C28H24FN3O5
|
|---|---|
| 分子量 |
501.51
|
| 精确质量 |
501.17
|
| 元素分析 |
C, 67.06; H, 4.82; F, 3.79; N, 8.38; O, 15.95
|
| CAS号 |
849217-68-1
|
| 相关CAS号 |
Cabozantinib S-malate;1140909-48-3;Cabozantinib-d6;1802168-46-2;Cabozantinib hydrochloride;1817759-42-4;Cabozantinib-d4;1802168-53-1
|
| PubChem CID |
25102847
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
758.1±60.0 °C at 760 mmHg
|
| 闪点 |
412.3±32.9 °C
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
| 折射率 |
1.688
|
| LogP |
4.84
|
| tPSA |
98.78
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
795
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1(CC1)C(NC1C=CC(OC2C3C(=CC(=C(C=3)OC)OC)N=CC=2)=CC=1)=O)NC1C=CC(F)=CC=1
|
| InChi Key |
ONIQOQHATWINJY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34)
|
| 化学名 |
1-N-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
|
| 别名 |
Cabozantinib; XL-184; BMS-907351; BMS907351; XL184; XL 184; BMS 907351; Cabozantinib free base; trade name Cometriq
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.98 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (4.15 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: ≥ 2.08 mg/mL (4.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 5 中的溶解度: 2% DMSO +30%PEG 300 +5% Tween 80 +ddH2O: 2mg/mL 配方 6 中的溶解度: 2.5 mg/mL (4.98 mM) in 0.5% CMC/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9940 mL | 9.9699 mL | 19.9398 mL | |
| 5 mM | 0.3988 mL | 1.9940 mL | 3.9880 mL | |
| 10 mM | 0.1994 mL | 0.9970 mL | 1.9940 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Cabozantinib Plus Pembrolizumab as First-Line Therapy for Cisplatin-Ineligible Advanced Urothelial Carcinoma
CTID: NCT03534804
Phase: Phase 2 Status: Completed
Date: 2024-11-26
|
The multi-tyrosine kinase inhibitor, XL184, targeting MET and VEGFR2 abrogates MPNST migration, invasion, and angiogenesis. Clin Cancer Res. 2011 Jun 15;17(12):3943-55. |
XL184 abrogates local and metastatic MPNST growth in vivo. Clin Cancer Res. 2011 Jun 15;17(12):3943-55. |