| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |

| 靶点 |
Histamine receptor
Histamine H1 receptor (H1R) (human H1R, Ki=0.32 nM; rat H1R, Ki=0.6 nM) [1] Histamine H2 receptor, muscarinic receptors, adrenergic receptors (Ki>1000 nM, negligible affinity) [1] |
|---|---|
| 体外研究 (In Vitro) |
西替利嗪仅轻微穿过血脑屏障,减少了旧抗组胺药常见的镇静副作用。它还被证明可以抑制嗜酸性粒细胞趋化性和 LTB4 释放。 Boone 等人的剂量为 20 毫克。发现它抑制特应性皮炎患者 VCAM-1 的表达。西替利嗪的左旋对映体称为左西替利嗪,是活性更强的形式。西替利嗪二盐酸盐是第二代抗组胺药,是羟嗪的主要代谢产物,也是一种外消旋选择性 H1 受体反向激动剂,用于治疗过敏、花粉热、血管性水肿和荨麻疹。西替利嗪仅轻微穿过血脑屏障,减少了老式抗组胺药常见的镇静副作用。它还被证明可以抑制嗜酸性粒细胞趋化性和 LTB4 释放。 Boone 等人的剂量为 20 毫克。发现它抑制特应性皮炎患者 VCAM-1 的表达。西替利嗪的左旋对映体称为左西替利嗪,是活性更强的形式。
表达人H1R的HEK293细胞膜组分的放射性配体结合实验证实,盐酸西替利嗪(Cetirizine DiHCl; P071)是高亲和力竞争性H1R拮抗剂,浓度依赖性置换[3H]-吡拉明[1] - TNF-α(10 ng/mL)刺激的人气道上皮细胞(HAECs)经盐酸西替利嗪(Cetirizine DiHCl; P071)(1 μM-50 μM)处理后,20 μM浓度时较单独TNF-α组减少58%的IL-8分泌和45%的GM-CSF分泌,机制与抑制NF-κB激活相关[2] - LPS(1 μg/mL)刺激的人外周血单个核细胞(PBMCs)经盐酸西替利嗪(Cetirizine DiHCl; P071)(0.1 μM-10 μM)处理后,10 μM浓度时抑制巨噬细胞迁移抑制因子(MIF)mRNA表达62%、MIF蛋白分泌55%,发挥H1R拮抗之外的抗炎作用[3] - 组胺(1 μM)预收缩的分离豚鼠气管平滑肌条经盐酸西替利嗪(Cetirizine DiHCl; P071)(0.1 μM-10 μM)处理后,药物呈浓度依赖性舒张平滑肌,EC50=1.2 μM[1] - 抗IgE(1 μg/mL)刺激的人外周血嗜碱性粒细胞经盐酸西替利嗪(Cetirizine DiHCl; P071)(1 nM-10 μM)处理后,10 μM浓度时抑制组胺释放70%,IC50=0.9 μM[1] |
| 体内研究 (In Vivo) |
西替利嗪(20 mg/kg,小鼠,口服)通过抑制豚草花粉免疫和攻击的小鼠中 MIF 和 IL-8 的产生来发挥抗炎作用。
大鼠被动皮肤过敏反应(PCA)模型:背部皮内注射抗卵清蛋白IgE(0.1 mL)的大鼠,48小时后口服灌胃盐酸西替利嗪(Cetirizine DiHCl; P071)(1 mg/kg、3 mg/kg、10 mg/kg),1小时后静脉注射卵清蛋白(1 mg/kg)+伊文思蓝(5 mg/kg)。30分钟后处死大鼠,测量皮肤风团面积,10 mg/kg剂量时抑制率达85%[1] - 豚鼠过敏性鼻炎模型:第0天和第7天腹腔注射卵清蛋白(100 μg)+氢氧化铝(2 mg)致敏豚鼠,第14天起每日鼻内给予卵清蛋白(1%溶液)攻击,每次攻击前1小时口服灌胃盐酸西替利嗪(Cetirizine DiHCl; P071)(5 mg/kg),连续7天。药物使喷嚏次数减少72%,鼻分泌物减少65%,同时鼻黏膜嗜酸性粒细胞浸润减少[1] - 慢性特发性荨麻疹患者临床试验:成人口服盐酸西替利嗪(Cetirizine DiHCl; P071)(10 mg/天)治疗6周,与安慰剂相比,风团数量减少75%,瘙痒程度减轻70%,症状缓解可持续24小时[1] - 小鼠LPS诱导炎症模型:腹腔注射LPS(5 mg/kg)诱导全身炎症前1小时,腹腔注射盐酸西替利嗪(Cetirizine DiHCl; P071)(10 mg/kg),LPS注射后6小时,血清MIF水平降低48%,TNF-α水平降低42%,证实体内抗炎活性[3] |
| 酶活实验 |
Cell Line: 人气道上皮细胞系A549
浓度: 0-10 μM 孵育时间: 24 h 结果: 不同浓度西替利嗪(0.1, 1, 2.5, 5)孵育A549细胞的存活情况、10μM)24小时经MTT测试与对照组相比均高于90%。 5 和 10 μM 西替利嗪分别抑制 GM-CSF 释放 70.71% 和 61.55%。与 10 μM 西替利嗪预孵育可抑制 75.04% 的 IL-8 分泌。 H1R结合实验:从表达人H1R的HEK293细胞或大鼠脑组织制备膜组分,将膜样品与[3H]-吡拉明(0.5 nM)及不同浓度的盐酸西替利嗪(Cetirizine DiHCl; P071)(0.01 nM-100 nM)在25°C孵育60分钟。通过真空过滤玻璃纤维滤膜分离结合态和游离态配体,用液体闪烁计数器测量放射性,采用Cheng-Prusoff方程计算Ki值[1] |
| 细胞实验 |
最近的研究表明,除了H(1)受体拮抗作用外,几种第二代抗组胺药还可以调节各种炎症反应。抗组胺药西替利嗪是左西替利辛和右西替利津的外消旋混合物。本研究的目的是研究这两种抗组胺药(西替利嗪和左西替利津)对A549人气道上皮细胞粒细胞-巨噬细胞集落刺激因子(GM-CSF)和白细胞介素(IL)-8分泌的影响。将A549细胞分别与西替利嗪(0.1、1、2.5、5和10μM)或左西替利津(0.1、2、5、和10μm)预孵育16小时,然后用IL-1β刺激8小时。采用酶联免疫吸附试验(ELISA)测定培养上清液中GM-CSF和IL-8的水平。我们的数据显示,西替利嗪(5和10微克)和左西替利津(2.5、5和10微米)显著抑制了IL-1β刺激的A549细胞的GM-CSF分泌(p<0.05)。在刺激A549后,西替利嗪(10μM)和左西替利津(5和10μm)显著抑制IL-8的分泌。2.5μM的左西替利嗪和5μM的西替利津,以及5μM左西替利嗪和10μM西替利津之间的抑制作用是可比较的。此外,5μM左西替利嗪在抑制IL-8分泌方面优于5μM西替利津,但在其他条件下没有出现这种差异。我们的结果表明,更高浓度的西替利嗪和左西替利津可以减少IL-1β刺激的A549细胞释放GM-CSF和IL-8。这些观察结果表明,两种第二代抗组胺药的抗炎作用可能超过组胺H(1)受体拮抗剂,左西替利嗪在这种活性方面发挥着重要作用[2]。
气道上皮细胞细胞因子分泌实验:将HAECs接种于24孔板,培养至80%融合,用盐酸西替利嗪(Cetirizine DiHCl; P071)(1 μM-50 μM)预处理1小时,再用TNF-α(10 ng/mL)刺激24小时。收集培养上清液,ELISA法量化IL-8和GM-CSF水平;提取核蛋白,Western blot检测NF-κB p65激活情况[2] - PBMC MIF表达实验:密度梯度离心法分离人PBMCs,接种于6孔板,用盐酸西替利嗪(Cetirizine DiHCl; P071)(0.1 μM-10 μM)预处理1小时,再用LPS(1 μg/mL)刺激18小时。提取总RNA,RT-PCR检测MIF mRNA水平;收集上清液,ELISA法测量MIF蛋白含量[3] - 嗜碱性粒细胞组胺释放实验:密度梯度离心法分离人外周血嗜碱性粒细胞,用缓冲液重悬后,加入盐酸西替利嗪(Cetirizine DiHCl; P071)(1 nM-10 μM)预处理30分钟,再用抗IgE(1 μg/mL)在37°C刺激60分钟。离心收集上清液,荧光法检测组胺浓度[1] - 气管平滑肌舒张实验:分离豚鼠气管条,置于含氧合Krebs-Ringer溶液(37°C,95% O2/5% CO2)的器官浴中平衡60分钟,用组胺(1 μM)预收缩后,累积加入盐酸西替利嗪(Cetirizine DiHCl; P071)(0.1 μM-10 μM),记录张力变化[1] |
| 动物实验 |
Male 8-week-old BALB/c mice (25-30 g) immunized and challenged with ragweed pollen
2 or 20 mg/kg Orally, diluted in sterile water on days 18, 19, and 20. PCA rat model: Male Wistar rats (150-200 g) were intradermally injected with anti-ovalbumin IgE (0.1 mL) on the back. After 48 hours, Cetirizine DiHCl (P071) was dissolved in physiological saline and administered via oral gavage (1 mg/kg, 3 mg/kg, 10 mg/kg). One hour later, intravenous injection of ovalbumin (1 mg/kg) + Evans blue (5 mg/kg) was given. Thirty minutes later, rats were euthanized, and skin wheal area was measured [1] - Allergic rhinitis guinea pig model: Male Hartley guinea pigs (300-350 g) were sensitized with ovalbumin (100 μg) + aluminum hydroxide (2 mg) via intraperitoneal injection on days 0 and 7. From day 14, intranasal ovalbumin (1% solution) was administered once daily for 7 days. Cetirizine DiHCl (P071) (5 mg/kg) was given via oral gavage once daily 1 hour before challenge. Record sneezing frequency and nasal secretion for 10 minutes post-challenge; collect nasal mucosa for eosinophil counting [1] - Murine LPS inflammation model: Male BALB/c mice (20-25 g) were intraperitoneally injected with LPS (5 mg/kg) to induce systemic inflammation. Cetirizine DiHCl (P071) (10 mg/kg) was injected intraperitoneally 1 hour before LPS administration. At 6 hours post-LPS injection, collect blood to measure serum MIF and TNF-α levels via ELISA [3] |
| 药代性质 (ADME/PK) |
Absorption: Oral bioavailability is 70-80% in humans; peak plasma concentration (Cmax) is reached at 1-2 hours post-oral administration (10 mg dose: Cmax=310 ng/mL) [1]
- Distribution: Volume of distribution (Vd) is 0.4-0.6 L/kg in humans; brain/plasma concentration ratio <0.01, indicating minimal blood-brain barrier penetration [1] - Metabolism: Minimally metabolized in the liver (<10% of dose), with >90% excreted as unchanged drug [1] - Excretion: 60% of the dose is excreted in urine (50% as unchanged drug, 10% as metabolites), 30% in feces. Elimination half-life (t1/2) is 7-10 hours in humans [1] - Plasma protein binding: < |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Small occasional doses of cetirizine are acceptable during breastfeeding. Larger doses or more prolonged use may cause drowsiness and other effects in the infant or decrease the milk supply, particularly in combination with a sympathomimetic such as pseudoephedrine or before lactation is well established. International guidelines recommend cetirizine as an acceptable choice if an antihistamine is required during breastfeeding. Cetirizine has been used successfully in cases of persistent pain of the breast during breastfeeding. Ophthalmic use of cetirizine by the mother should pose little risk to the breastfed infant. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants In one telephone follow-up study, mothers reported irritability and colicky symptoms 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention. A woman who was nursing (extent not stated) her newborn infant was treated for pemphigus with oral prednisolone 25 mg daily, with the dosage increased over 2 weeks to 60 mg daily. She was also taking cetirizine 10 mg daily and topical betamethasone 0.1% twice daily to the lesions. Because of a poor response, the betamethasone was changed to clobetasol propionate ointment 0.05%. She continued breastfeeding throughout treatment and her infant was developing normally at 8 weeks of age and beyond. A woman with narcolepsy took sodium oxybate 4 grams each night at 10 pm and 2 am as well as fluoxetine 20 mg and cetirizine 5 mg daily throughout pregnancy and postpartum. She breastfed her infant except for 4 hours after the 10 pm oxybate dose and 4 hours after the 2 am dose. She either pumped breastmilk or breastfed her infant just before each dose of oxybate. The infant was exclusively breastfed or breastmilk fed for 6 months when solids were introduced. The infant was evaluated at 2, 4 and 6 months with the Ages and Stages Questionnaires, which were withing the normal range as were the infant's growth and pediatrician's clinical impressions regarding the infant's growth and development. Three women taking long-term cetirizine 10 mg daily by mouth while exclusively breastfeeding their 5- to 6-month old infants. The mothers reported no adverse effects in their infants. Thirty-one women taking cetirizine 10 mg (n = 29) or 20 mg (n = 2) daily reported no adverse effects in 61% of their infants and minor adverse effects fever, sedation, rash, poor feeding, bruising, refusing of the breast or constipation. But mothers attributed these effects to other causes such a cold, weaning or learning to crawl. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. Whether lower oral doses of cetirizine have the same effect on serum prolactin or whether the effects on prolactin have any consequences on breastfeeding success have not been studied. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. In a study of 31 women taking cetirizine 10 mg (n = 29) or 20 mg (n = 2) daily, 10 reported a perceived decrease in milk supply over the prior 3 days. Acute toxicity: LD50 is >2000 mg/kg (oral) in rats and mice; no mortality or severe clinical signs (convulsions, respiratory depression) reported [1] - Chronic toxicity: Rats administered Cetirizine DiHCl (P071) (100 mg/kg/day, oral) for 6 months showed no significant liver/kidney toxicity, hematological abnormalities, or organ weight changes [1] - Clinical side effects: Mild drowsiness (5-10% of patients), headache (4-6%), and dry mouth (2-3%) are reported. Sedative effects are minimal due to low blood-brain barrier penetration [1] - Drug-drug interaction: No significant interaction with CYP450 isoform substrates or inhibitors; no potentiation of CNS depressant effects when co-administered with alcohol or benzodiazepines [1] |
| 参考文献 | |
| 其他信息 |
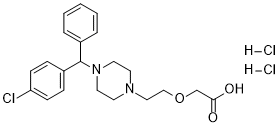
Cetirizine hydrochloride is a diarylmethane.
Cetirizine Hydrochloride is a synthetic phenylmethyl-piperazinyl derivative, antihistaminic Cetirizine is a metabolite of hydroxyzine and a selective peripheral histamine H1-receptor antagonist. It is used for symptomatic treatment of seasonal and perennial allergic rhinitis and for chronic urticaria. (NCI04) A potent second-generation histamine H1 antagonist that is effective in the treatment of allergic rhinitis, chronic urticaria, and pollen-induced asthma. Unlike many traditional antihistamines, it does not cause drowsiness or anticholinergic side effects. See also: Cetirizine (has active moiety) ... View More ... Cetirizine DiHCl (P071) is a second-generation, non-sedating histamine H1 receptor antagonist with dual anti-allergic and anti-inflammatory activities [1,2,3] Its core mechanism is competitive H1R antagonism, blocking histamine-mediated allergic responses (vascular hyperpermeability, smooth muscle contraction, histamine release) [1] Beyond H1R blockade, it suppresses pro-inflammatory cytokines (IL-8, GM-CSF) and macrophage migration inhibitory factor (MIF) expression, enhancing anti-inflammatory efficacy [2,3] Indications include seasonal/perennial allergic rhinitis (relieving sneezing, rhinorrhea, nasal itching) and chronic idiopathic urticaria (reducing wheal and pruritus) [1] Minimal blood-brain barrier penetration and high H1R selectivity distinguish it from first-generation antihistamines, with reduced sedative side effects [1] It exhibits rapid onset of action (within 1 hour post-administration) and sustained efficacy, supporting once-daily dosing (10 mg) for adults [1] |
| 分子式 |
C21H27CL3N2O3
|
|
|---|---|---|
| 分子量 |
461.81
|
|
| 精确质量 |
460.11
|
|
| 元素分析 |
C, 54.62; H, 5.89; Cl, 23.03; N, 6.07; O, 10.39
|
|
| CAS号 |
83881-52-1
|
|
| 相关CAS号 |
Cetirizine; 83881-51-0; Cetirizine-d4; 1219803-84-5; Cetirizine-d8; 774596-22-4; Levocetirizine; 130018-77-8; Levocetirizine dihydrochloride; 130018-87-0; Cetirizine methyl ester; 83881-46-3; Cetirizine-d4 dihydrochloride; Cetirizine-d8 dihydrochloride; 2070015-04-0
|
|
| PubChem CID |
55182
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.237 g/cm3
|
|
| 沸点 |
542.1ºC at 760 mmHg
|
|
| 熔点 |
110-115ºC
|
|
| 闪点 |
281.6ºC
|
|
| LogP |
3.826
|
|
| tPSA |
53.01
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
443
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
Cl.O=C(COCCN1CCN(C(C2C=CC(Cl)=CC=2)C2C=CC=CC=2)CC1)O
|
|
| InChi Key |
PGLIUCLTXOYQMV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H25ClN2O3.2ClH/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26;;/h1-9,21H,10-16H2,(H,25,26);2*1H
|
|
| 化学名 |
2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]acetic acid;dihydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 120 mg/mL (259.85 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1654 mL | 10.8270 mL | 21.6539 mL | |
| 5 mM | 0.4331 mL | 2.1654 mL | 4.3308 mL | |
| 10 mM | 0.2165 mL | 1.0827 mL | 2.1654 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。