| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
Antiviral; Plasmodium; SARS-COV-2; Malaria; TLRs; HIV-1
|
|---|---|
| 体外研究 (In Vitro) |
当暴露于 20 μM 氯喹 (CHQ) 时,活化的人单核细胞衍生的朗格汉斯样细胞 (MoLC) 的 Th1 启动能力降低,并且释放 IL-12p70 的能力较差。氯喹 (20 μM) 同时刺激 CD4+ T 细胞释放 IL-17A,并改善 MoLC 中 IL-1 诱导的 IL-23 [1]。在亲本 MDA-MB-231 细胞中,在常氧和缺氧条件下,25 μM 氯喹都会抑制 MMP-9 mRNA 表达。氯喹对 MMP-2、MMP-9 和 MMP-13 mRNA 的影响取决于细胞表达、剂量和缺氧情况 [2]。当用 IRS-954 或氯喹抑制 TLR7 和 TLR9 时,体外观察到 HuH7 细胞增殖显着减少 [3]。在低微摩尔剂量(EC50=1.13 μM)下,氯喹(0.01–100 μM;48 小时)可抑制 SARS-CoV-2 感染,并有效阻断 vero E6 细胞中的病毒感染。通过提高病毒/细胞融合所需的内体 pH 值并干扰 SARS-CoV 细胞获得的糖基化,氯喹引起病毒感染 [4]。
|
| 体内研究 (In Vivo) |
在原位小鼠模型中,氯喹(80 mg/kg,腹腔注射)不会阻止三阴性 MDA-MB-231 细胞生长,无论 TLR9 表达多少[2]。 IRS-954 或氯喹诱导的 TLR7 和 TLR9 抑制显着降低肿瘤异种移植模型的生长。此外,在DEN/NMOR肿瘤模型中,氯喹大大减少了HCC的形成[3]。
|
| 酶活实验 |
氯喹抑制基质金属蛋白酶(MMP)-2和MMP-9的mRNA表达和蛋白活性,而MMP-13的mRNA表达及蛋白水解活性增加。尽管氯喹增强了TLR9 mRNA的表达,但在体外却抑制了TLR9蛋白的表达。[2]
|
| 细胞实验 |
在本研究中,我们研究了CHQ对人单核细胞衍生的郎格汉斯样细胞(MoLC)和树突状细胞(MoDC)对IL-1β的反应的影响。CHQ的存在减少了两个亚群中IL-12p70的释放,但令人惊讶地增加了MoDC中IL-6的产生和MoLC中IL-23的产生。重要的是,CHQ处理的MoLC促进CD4(+)T细胞分泌IL-17A,并提高RORC mRNA水平,而IFN-γ的释放减少。MoLC和MoDC中IL-12家族细胞因子的失调发生在转录水平。其他晚期自噬抑制剂也获得了类似的效果,而PI3K抑制剂3-甲基腺嘌呤不能增加IL-23的分泌。调节的细胞因子释放依赖于IL-1细胞因子的激活,并被特异性IL-1R拮抗剂消除。CHQ升高了TNFR相关因子6的表达,该因子是IL-1R和TLR依赖性信号传导中的常见中间体。因此,用Pam3CSK4和CHQ处理增强了MoLC和MoDC中IL-23的释放。CHQ抑制自噬流量,通过增加LC3-II和p62的表达证实,并激活ERK、p38和JNK-MAPK,但仅抑制p38消除了MoLC释放的IL-23。因此,我们的研究结果表明,CHQ以p38依赖的方式调节细胞因子的释放,这表明郎格罕细胞和树突状细胞在CHQ引发的银屑病中发挥着重要作用,可能是通过促进Th17免疫。[1]
|
| 动物实验 |
Control and TLR9 siRNA MDA-MB-231 cells (5×105 cells in 100 μl) were inoculated into the mammary fat pads of four-week-old, immune-deficient mice (athymic nude/nu Foxn1; Harlan Sprague Dawley, Inc., Indianapolis, IN, USA). Treatments were started seven days after tumor cell inoculation. The mice were treated daily either with intraperitoneal (i.p.) chloroquine (80 mg/kg) or vehicle (PBS). The animals were monitored daily for clinical signs. Tumor measurements were performed twice a week and tumor volume was calculated according to the formula V = (π / 6) (d1 × d2)3/2, where d1 and d2 are perpendicular tumor diameters (9). The tumors were allowed to grow for 22 days, at which point the mice were sacrificed and the tumors were dissected for a final measurement. Throughout the experiments, the animals were maintained under controlled pathogen-free environmental conditions (20–21ºC, 30–60% relative humidity and a 12-h lighting cycle). The mice were fed with small-animal food pellets (Harlan Sprague Dawley) and supplied with sterile water ad libitum. The experimental procedures were reviewed and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.[2]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Chloroquine oral solution has a bioavailability of 52-102% and oral tablets have a bioavailability of 67-114%. Intravenous chloroquine reaches a Cmax of 650-1300µg/L and oral chloroquine reaches a Cmax of 65-128µg/L with a Tmax of 0.5h. Chloroquine is predominantly eliminated in the urine. 50% of a dose is recovered in the urine as unchanged chloroquine, with 10% of the dose recovered in the urine as desethylchloroquine. The volume of distribution of chloroquine is 200-800L/kg. Chloroquine has a total plasma clearance of 0.35-1L/h/kg. Chloroquine is rapidly and almost completely absorbed from the GI tract following oral administration, and peak plasma concn of the drug are generally attained within 1-2 hr. Considerable interindividual variations in serum concn of chloroquine have been reported. Oral administration of 310 mg of chloroquine daily reportedly results in peak plasma concn of about 0.125 ug/mL. If 500 mg of chloroquine is administered once weekly, peak plasma concn of the drug reportedly range from 0.15-0.25 ug/mL and trough plasma concn reportedly range from 0.02-0.04 ug/mL. Results of one study indicate that chloroquine may exhibit nonlinear dose dependent pharmacokinetics. In this study, administration of a single 500 mg oral dose of chloroquine resulted in a peak serum concentration of 0.12 ug/mL, and administration of a single 1 g oral dose of the drug resulted in a peak serum concentration of 0.34 ug/mL. Results of one cross-over study in healthy adults indicate that the bioavailability of chloroquine is greater when the drug is administered with food than when the drug is administered in the fasting state. In this study, the rate of absorption of chloroquine was unaffected by the presence of food in the GI tract however, peak plasma concn of chloroquine and areas under the plasma concentration-time curves were higher when 600 mg of the drug was administered with food than when the same dose was administered without food. Chloroquine is widely distributed into body tissues. The drug has an apparent volume of distribution of 116-285 L/kg in healthy adults. Animal studies indicate that concn of chloroquine in liver, spleen, kidney, and lung are at least 200-700 times higher than those in plasma, and concentration of the drug in brain and spinal cord are at least 10-30 times higher than those in plasma. Chloroquine binds to melanin containing cells in the eyes and skin; skin concn of the drug are considerably higher than plasma concentration. Animal studies indicate that the drug is concentrated in the iris and choroid and, to a lesser extent, in the cornea, retina, and sclera and is found in these tissues in higher concentration than in other tissues. Chloroquine is also concentrated in erythrocytes and binds to platelets and granulocytes. Serum concentrations of chloroquine are higher than those in plasma, presumably because the drug is released from platelets during coagulation, and plasma concentrations are 10 to 15% lower than whole blood concentration of the drug. For more Absorption, Distribution and Excretion (Complete) data for CHLOROQUINE (16 total), please visit the HSDB record page. Metabolism / Metabolites Chloroquine is N-dealkylated primarily by CYP2C8 and CYP3A4 to N-desethylchloroquine. It is N-dealkylated to a lesser extent by CYP3A5, CYP2D6, and to an ever lesser extent by CYP1A1. N-desethylchloroquine can be further N-dealkylated to N-bidesethylchloroquine, which is further N-dealkylated to 7-chloro-4-aminoquinoline. Chloroquine is partially metabolized; the major metabolite is desethylchloroquine. Desethylchloroquine also has antiplasmodial activity, but is slightly less active than chloroquine. Bisdesethylchloroquine, which is a carboxylic acid derivative, and several other unidentified metabolites are also formed in small amounts. Hepatic (partially), to active de-ethylated metabolites. Principal metabolite is desethylchloroquine Completely absorbed from gastrointestinal tract. Chloroquine is partially metabolized; the major metabolite is desethylchloroquine. Desethylchloroquine also has antiplasmodial activity, but is slightly less active than chloroquine. Bisdesethylchloroquine, which is a carboxylic acid derivative, and several other unidentified metabolites are also formed in small amounts (A625). Route of Elimination: Excretion of chloroquine is quite slow, but is increased by acidification of the urine. Half Life: 1-2 months Biological Half-Life The half life of chloroquine is 20-60 days. The plasma half-life of chloroquine in healthy individuals is generally reported to be 72-120 hr. In one study, serum concentrations of chloroquine appeared to decline in a biphasic manner and the serum half-life of the terminal phase increased with higher dosage of the drug. In this study, the terminal half-life of chloroquine was 3.1 hr after a single 250 mg oral dose, 42.9 hr after a single 500 mg oral dose, and 312 hr after a single 1 g oral dose of the drug. Terminal elimination half-life is 1 to 2 months. ... extremely slow elimination, with a terminal elimination half-life of 200 to 300 hours) |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite use for more than 50 years, chloroquine has rarely been linked to serum aminotransferase elevations or to clinically apparent acute liver injury. In patients with acute porphyria and porphyria cutanea tarda, chloroquine can trigger an acute attack with fever and serum aminotransferase elevations, sometimes resulting in jaundice. Hydroxychloroquine does not cause this reaction and appears to have partial beneficial effects in porphyria. In clinical trials of chloroquine for COVID-19 prevention and treatment, there were no reports of hepatotoxicity, and rates of serum enzyme elevations during chloroquine treatment were low and similar to those in patients receiving placebo or standard of care. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Very small amounts of chloroquine are excreted in breast milk; when given once weekly, the amount of drug is not sufficient to harm the infant nor is the quantity sufficient to protect the child from malaria. United Kingdom malaria treatment guidelines recommend that weekly chloroquine 500 mg be given until breastfeeding is completed and primaquine can be given. Breastfeeding infants should receive the recommended dosages of chloroquine for malaria prophylaxis.In HIV-infected women, elevated viral HIV loads in milk were decreased after treatment with chloroquine to a greater extent than other women who were treated with the combination of sulfadoxine and pyrimethamine. Because no information is available on the daily use of chloroquine during breastfeeding, hydroxychloroquine or another agent may be preferred in this situation, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Several authors have pointed out that malaria prophylaxis in nursing mothers with chloroquine is common in endemic areas. As of the revision date, no reports of adverse reactions in breastfed infants have been published. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Chloroquine is 46-74% bound to plasma proteins. (-)-chloroquine binds more strongly to alpha-1-acid glycoprotein and (+)-chloroquine binds more strongly to serum albumin. |
| 参考文献 |
[1]. Said A, et al. Chloroquine promotes IL-17 production by CD4+ T cells via p38-dependent IL-23 release by monocyte-derived Langerhans-like cells. J Immunol. 2014 Dec 15;193(12):6135-43.
[2]. Tuomela J, et al. Chloroquine has tumor-inhibitory and tumor-promoting effects in triple-negative breast cancer. Oncol Lett. 2013 Dec;6(6):1665-1672. [3]. Mohamed FE, et al. Effect of toll-like receptor 7 and 9 targeted therapy to prevent the development of hepatocellular carcinoma. Liver Int. 2014 Jul 2. doi: 10.1111/liv.12626. [4]. Colson P, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. [5]. Savarino A, et al. The anti-HIV-1 activity of chloroquine. J Clin Virol. 2001;20(3):131-135. |
| 其他信息 |
Chloroquine is an aminoquinoline that is quinoline which is substituted at position 4 by a [5-(diethylamino)pentan-2-yl]amino group at at position 7 by chlorine. It is used for the treatment of malaria, hepatic amoebiasis, lupus erythematosus, light-sensitive skin eruptions, and rheumatoid arthritis. It has a role as an antimalarial, an antirheumatic drug, a dermatologic drug, an autophagy inhibitor and an anticoronaviral agent. It is an aminoquinoline, a secondary amino compound, a tertiary amino compound and an organochlorine compound. It is a conjugate base of a chloroquine(2+).
Chloroquine is an aminoquinolone derivative first developed in the 1940s for the treatment of malaria. It was the drug of choice to treat malaria until the development of newer antimalarials such as [pyrimethamine], [artemisinin], and [mefloquine]. Chloroquine and its derivative [hydroxychloroquine] have since been repurposed for the treatment of a number of other conditions including HIV, systemic lupus erythematosus, and rheumatoid arthritis. **The FDA emergency use authorization for [hydroxychloroquine] and chloroquine in the treatment of COVID-19 was revoked on 15 June 2020.** Chloroquine was granted FDA Approval on 31 October 1949. Chloroquine is an Antimalarial. Chloroquine is an aminoquinoline used for the prevention and therapy of malaria. It is also effective in extraintestinal amebiasis and as an antiinflammatory agent for therapy of rheumatoid arthritis and lupus erythematosus. Chloroquine is not associated with serum enzyme elevations and is an extremely rare cause of clinically apparent acute liver injury. Chloroquine has been reported in Cocos nucifera, Cinchona calisaya, and other organisms with data available. Chloroquine is a 4-aminoquinoline with antimalarial, anti-inflammatory, and potential chemosensitization and radiosensitization activities. Although the mechanism is not well understood, chloroquine is shown to inhibit the parasitic enzyme heme polymerase that converts the toxic heme into non-toxic hemazoin, thereby resulting in the accumulation of toxic heme within the parasite. This agent may also interfere with the biosynthesis of nucleic acids. Chloroquine's potential chemosensitizing and radiosensitizing activities in cancer may be related to its inhibition of autophagy, a cellular mechanism involving lysosomal degradation that minimizes the production of reactive oxygen species (ROS) related to tumor reoxygenation and tumor exposure to chemotherapeutic agents and radiation. Chloroquine is only found in individuals that have used or taken this drug. It is a prototypical antimalarial agent with a mechanism that is not well understood. It has also been used to treat rheumatoid arthritis, systemic lupus erythematosus, and in the systemic therapy of amebic liver abscesses. [PubChem]The mechanism of plasmodicidal action of chloroquine is not completely certain. Like other quinoline derivatives, it is thought to inhibit heme polymerase activity. This results in accumulation of free heme, which is toxic to the parasites. nside red blood cells, the malarial parasite must degrade hemoglobin to acquire essential amino acids, which the parasite requires to construct its own protein and for energy metabolism. Digestion is carried out in a vacuole of the parasite cell.During this process, the parasite produces the toxic and soluble molecule heme. The heme moiety consists of a porphyrin ring called Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule, the parasite biocrystallizes heme to form hemozoin, a non-toxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.Chloroquine enters the red blood cell, inhabiting parasite cell, and digestive vacuole by simple diffusion. Chloroquine then becomes protonated (to CQ2+), as the digestive vacuole is known to be acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine caps hemozoin molecules to prevent further biocrystallization of heme, thus leading to heme buildup. Chloroquine binds to heme (or FP) to form what is known as the FP-Chloroquine complex; this complex is highly toxic to the cell and disrupts membrane function. Action of the toxic FP-Chloroquine and FP results in cell lysis and ultimately parasite cell autodigestion. In essence, the parasite cell drowns in its own metabolic products. The prototypical antimalarial agent with a mechanism that is not well understood. It has also been used to treat rheumatoid arthritis, systemic lupus erythematosus, and in the systemic therapy of amebic liver abscesses. See also: Chloroquine Phosphate (has salt form); Chloroquine Sulfate (has salt form); Chloroquine Hydrochloride (has salt form) ... View More ... Drug Indication Chloroquine is indicated to treat infections of _P. vivax_, _P. malariae_, _P. ovale_, and susceptible strains of _P. falciparum_. It is also used to treat extraintestinal amebiasis. Chloroquine is also used off label for the treatment of rheumatic diseases, as well as treatment and prophylaxis of Zika virus. Chloroquine is currently undergoing clinical trials for the treatment of COVID-19. FDA Label Mechanism of Action Chloroquine inhibits the action of heme polymerase in malarial trophozoites, preventing the conversion of heme to hemazoin. _Plasmodium_ species continue to accumulate toxic heme, killing the parasite. Chloroquine passively diffuses through cell membranes and into endosomes, lysosomes, and Golgi vesicles; where it becomes protonated, trapping the chloroquine in the organelle and raising the surrounding pH. The raised pH in endosomes, prevent virus particles from utilizing their activity for fusion and entry into the cell. Chloroquine does not affect the level of ACE2 expression on cell surfaces, but inhibits terminal glycosylation of ACE2, the receptor that SARS-CoV and SARS-CoV-2 target for cell entry. ACE2 that is not in the glycosylated state may less efficiently interact with the SARS-CoV-2 spike protein, further inhibiting viral entry. The exact mechanism of antimalarial activity of chloroquine has not been determined. The 4-aminoquinoline derivatives appear to bind to nucleoproteins and interfere with protein synthesis in susceptible organisms; the drugs intercalate readily into double-stranded DNA and inhibit both DNA and RNA polymerase. In addition, studies using chloroquine indicate that the drug apparently concentrates in parasite digestive vacuoles, increases the pH of the vacuoles, and interferes with the parasite's ability to metabolize and utilize erythrocyte hemoglobin. Plasmodial forms that do not have digestive vacuoles and do not utilize hemoglobin, such as exoerythrocytic forms, are not affected by chloroquine. The 4-aminoquinoline derivatives, including chloroquine, also have anti-inflammatory activity; however, the mechanism(s) of action of the drugs in the treatment of rheumatoid arthritis and lupus erythematosus has not been determined. Chloroquine reportedly antagonizes histamine in vitro, has antiserotonin effects, and inhibits prostaglandin effects in mammalian cells presumably by inhibiting conversion of arachidonic acid to prostaglandin F2. In vitro studies indicate that chloroquine also inhibits chemotaxis of polymorphonuclear leukocytes, macrophages, and eosinophils. Antiprotozoal-Malaria: /Mechanism of action/ may be based on ability of chloroquine to bind and alter the properties of DNA. Chloroquine also is taken up into the acidic food vacuoles of the parasite in the erythrocyte. It increases the pH of the acid vesicles, interfering with vesicle functions and possibly inhibiting phospholipid metabolism. In suppressive treatment, chloroquine inhibits the erythrocytic stage of development of plasmodia. In acute attacks of malaria, chloroquine interrupts erythrocytic schizogony of the parasite. its ability to concentrate in parasitized erythrocytes may account for its selective toxicity against the erythrocytic stages of plasmodial infection. Antirheumatic-Chloroquine is though to act as a mild immunosuppressant, inhibiting the production of rheumatoid factor and acute phase reactants. It also accumulates in white blood cells, stabilizing lysosomal membranes and inhibiting the activity of many enzymes, including collagenase and the proteases that cause cartilage breakdown. |
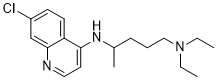
| 分子式 |
C18H26CLN3
|
|---|---|
| 分子量 |
319.87
|
| 精确质量 |
319.181
|
| 元素分析 |
C, 67.59; H, 8.19; Cl, 11.08; N, 13.14
|
| CAS号 |
54-05-7
|
| 相关CAS号 |
Chloroquine phosphate;50-63-5;Chloroquine-d5;1854126-41-2;Chloroquine dihydrochloride;3545-67-3;Chloroquine-d5 diphosphate; 132-73-0 (sulfate); 1854126-42-3; 54-05-7 ;151-69-9 (acetate) ; 1446-17-9 (phosphate); 3545-67-3 (HCl) ; 50-63-5 (diphosphate) ;
|
| PubChem CID |
2719
|
| 外观&性状 |
WHITE TO SLIGHTLY YELLOW, CRYSTALLINE POWDER
Colorless crystals |
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
460.6±40.0 °C at 760 mmHg
|
| 熔点 |
87ºC
|
| 闪点 |
232.3±27.3 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.592
|
| LogP |
4.69
|
| tPSA |
28.16
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
309
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C2C(C=1[H])=NC([H])=C([H])C=2N([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])[H]
|
| InChi Key |
WHTVZRBIWZFKQO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21)
|
| 化学名 |
N4-(7-chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine
|
| 别名 |
RP 3377; RP-3377; RP3377;Imagon; NSC 187208; NSC-187208; NSC187208;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol : ~100 mg/mL (~312.63 mM)
DMSO : ~50 mg/mL (~156.31 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.82 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.82 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (31.26 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 需要超声助溶并加热至 44°C。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1263 mL | 15.6314 mL | 31.2627 mL | |
| 5 mM | 0.6253 mL | 3.1263 mL | 6.2525 mL | |
| 10 mM | 0.3126 mL | 1.5631 mL | 3.1263 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。