| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
mGlu5 receptor (IC50 = 2.2 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在稳定表达人 mGlu5 的 HEK293 细胞中,CTEP (RO 4956371) 抑制君子氨酸诱导的 Ca2+ 动员,IC50 为 11.4 nM,并抑制 [3H]IP 积聚,IC50 为 6.4 nM。在稳定表达人 mGlu5 的 HEK293 细胞中,CTEP (RO 4956371) 可抑制人 mGlu5 的组成活性大约 50%,IC50 为 40.1 nM[1]。
|
||
| 体内研究 (In Vivo) |
在接受焦虑治疗的小鼠中,CTEP (RO 4956371) 在 0.1 mg/kg 和 0.3 mg/kg 剂量下显着有效。在 Vogel 冲突饮酒测试中,CTEP (RO 4956371) 在较低剂量下没有影响,但在 0.3 和 1.0 mg/kg 时显着延长饮酒时间。小鼠中基于血浆和全脑匀浆中总药物浓度的 B/P 比为 2.6,而 CTEP (RO 4956371)(口服)的半衰期为 18 小时。将 CTEP (RO 4956371) 以生理盐水/吐温载体中的微悬浮液形式单次口服剂量 4.5 和 8.7 mg/kg 给予成年 C57BL/6 小鼠后,CTEP (RO 4956371) 被迅速吸收,并在约 30 分钟内达到接近最大暴露量。成年小鼠以每 48 小时口服 2 mg/kg 的剂量长期服用两个月,其脑部暴露的最低 CTEP (RO 4956371) 为 240 ng/g。 CTEP (RO 4956371) 在 mGlu5 表达已知的小鼠大脑区域中完全取代 [3H]ABP688,在全脑匀浆中评估时,导致平均化合物浓度为 77.5 ng/g 的剂量可以实现 50% 的取代[1]。在小鼠中,使用 CTEP(RO 4956371;2 mg/kg,po bid)每 48 小时可实现连续 mGlu5 占据。 Fmr1 敲除小鼠的海马长期抑郁加剧、蛋白质合成过多和听源性癫痫发作可通过 CTEP (RO 4956371)(2 mg/kg,口服)治疗得到纠正[2]。
|
||
| 酶活实验 |
对于所有滤过性放射性配体结合试验,表达目标受体或受体组合的膜制剂在放射性配体结合缓冲液(15 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, 1.25 mM CaCl2和1.25 mM MgCl2, pH 7.4)中重悬,膜悬液与适当浓度的放射性配体和未标记药物混合在96孔板中,总量为200 μL,在适当温度下孵育60分钟。在孵育结束时,将膜过滤到Whatman unfilter上,用0.1%聚乙烯亚胺在ish缓冲液(50 mM tris - hcl, pH 7.4)中预孵育,用Filtermate 196收集机洗涤,并用冰冷的tris缓冲液洗涤三次。每孔加入45 μL MicroScint 40,振荡20 min后,在Topcount微孔板闪烁计数器上定量过滤上捕获的放射性。在中试实验中确定每个检测的膜浓度和孵育时间。[1]
|
||
| 动物实验 |
|
||
| 参考文献 | |||
| 其他信息 |
The metabotropic glutamate receptor 5 (mGlu5) is a glutamate-activated class C G protein-coupled receptor widely expressed in the central nervous system and clinically investigated as a drug target for a range of indications, including depression, Parkinson's disease, and fragile X syndrome. Here, we present the novel potent, selective, and orally bioavailable mGlu5 negative allosteric modulator with inverse agonist properties 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine (CTEP). CTEP binds mGlu5 with low nanomolar affinity and shows >1000-fold selectivity when tested against 103 targets, including all known mGlu receptors. CTEP penetrates the brain with a brain/plasma ratio of 2.6 and displaces the tracer [(3)H]3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-methyl-oxime (ABP688) in vivo in mice from brain regions expressing mGlu5 with an average ED(50) equivalent to a drug concentration of 77.5 ng/g in brain tissue. This novel mGlu5 inhibitor is active in the stress-induced hyperthermia procedure in mice and the Vogel conflict drinking test in rats with minimal effective doses of 0.1 and 0.3 mg/kg, respectively, reflecting a 30- to 100-fold higher in vivo potency compared with 2-methyl-6-(phenylethynyl)pyridine (MPEP) and fenobam. CTEP is the first reported mGlu5 inhibitor with both long half-life of approximately 18 h and high oral bioavailability allowing chronic treatment with continuous receptor blockade with one dose every 48 h in adult and newborn animals. By enabling long-term treatment through a wide age range, CTEP allows the exploration of the full therapeutic potential of mGlu5 inhibitors for indications requiring chronic receptor inhibition.[1]
Fragile X syndrome (FXS) is the most common form of inherited intellectual disability. Previous studies have implicated mGlu5 in the pathogenesis of the disease, but a crucial unanswered question is whether pharmacological mGlu5 inhibition is able to reverse an already established FXS phenotype in mammals. Here we have used the novel, potent, and selective mGlu5 inhibitor CTEP to address this issue in the Fmr1 knockout mouse. Acute CTEP treatment corrects elevated hippocampal long-term depression, protein synthesis, and audiogenic seizures. Chronic treatment that inhibits mGlu5 within a receptor occupancy range of 81% ± 4% rescues cognitive deficits, auditory hypersensitivity, aberrant dendritic spine density, overactive ERK and mTOR signaling, and partially corrects macroorchidism. This study shows that a comprehensive phenotype correction in FXS is possible with pharmacological intervention starting in young adulthood, after development of the phenotype. It is of great interest how these findings may translate into ongoing clinical research testing mGlu5 inhibitors in FXS patients.[2] |
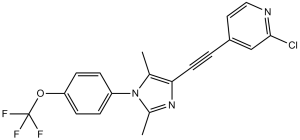
| 分子式 |
C19H13CLF3N3O
|
|
|---|---|---|
| 分子量 |
391.77
|
|
| 精确质量 |
391.07
|
|
| 元素分析 |
C, 58.25; H, 3.34; Cl, 9.05; F, 14.55; N, 10.73; O, 4.08
|
|
| CAS号 |
871362-31-1
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11646823
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| LogP |
4.835
|
|
| tPSA |
39.94
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
568
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
GOHCTCOGYKAJLZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H13ClF3N3O/c1-12-17(8-3-14-9-10-24-18(20)11-14)25-13(2)26(12)15-4-6-16(7-5-15)27-19(21,22)23/h4-7,9-11H,1-2H3
|
|
| 化学名 |
2-chloro-4-[2-[2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]imidazol-4-yl]ethynyl]pyridine
|
|
| 别名 |
RO-4956371; CTEP; RO4956371; 871362-31-1; 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine; mGluR5 inhibitor; CTEP (RO4956371); 2-chloro-4-[2-[2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]imidazol-4-yl]ethynyl]pyridine; E3BWG5775S; CHEMBL3410223; RO 4956371;
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.38 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.38 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W: 6 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5525 mL | 12.7626 mL | 25.5252 mL | |
| 5 mM | 0.5105 mL | 2.5525 mL | 5.1050 mL | |
| 10 mM | 0.2553 mL | 1.2763 mL | 2.5525 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03269331 | Completed | Behavioral: CTEP EBP Immersion | Evidence-Based Practice Nurse's Role |
David Grant U.S. Air Force Medical Center | September 15, 2017 | |
| NCT00093457 | Completed | Drug: sorafenib tosylate | Prostate Cancer | NCIC Clinical Trials Group | August 10, 2004 | Phase 2 |
| NCT01039155 | August 10, 2004 | Drug: Azacitidine Other: Laboratory Biomarker Analysis Drug: Oxaliplatin Other: Pharmacological Study |
Adult Solid Neoplasm Hematopoietic and Lymphoid Cell Neoplasm |
National Cancer Institute (NCI) | December 2009 | Phase 1 |
| NCT01281852 | Completed | Drug: Cisplatin Other: Laboratory Biomarker Analysis Drug: Paclitaxel Drug: Veliparib |
Cervical Adenocarcinoma Cervical Adenosquamous Carcinoma Cervical Squamous Cell Carcinoma, Not Otherwise Specified |
National Cancer Institute (NCI) | March 14, 2011 | Phase 1 |
| NCT00117169 | Completed | Procedure: multi-detector helical computed tomography |
Pulmonary Embolism | University Hospital, Geneva | January 2005 | Not Applicable |