Tricor, Procetofen, LF-178, Lipanthyl, Normalip, Secalip; Fenofibrate; LF 178, LF178,Controlip, durafenat
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Fenofibrate 具有相当程度的抑制 CYP2B6 (IC50=0.7±0.2 μM) 和 CYP2C19 (IC50=0.2±0.1 μM) 的效力。根据参考文献[1],非诺贝特对CYP2C8(IC50=4.8±1.7 μM)和CYP2C9(IC50=9.7 μM)具有中等抑制作用。与 PPARα 相比,非诺贝特对细胞色素 P450 环氧化酶 (CYP)2C 具有更大的亲和力并对其进行抑制。非诺贝特是一种众所周知的 PPARα 激动剂,但根据对 209 种常用药物和相关异生素的体外评估,它似乎也是细胞色素 P450 环氧化酶 (CYP)2C 的强抑制剂。与 PPARα (EC50=30 μM) 相比,非诺贝特对 CYP2C 的亲和力 (EC50=2.39±0.4 μM) 大于 10 倍。低剂量的非诺贝特会降低 CYP2C8 活性而不激活 PPARα[2]。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
在 10 μg/g/天的适度剂量下,非诺贝特每天可分别抑制 CYP2C8 过度表达诱导的视网膜和脉络膜新生血管形成 29% (P=0.021) 和 36% (P=1.2×10−9)。 2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A single 300mg oral dose of fenofibrate reaches a Cmax of 6-9.5mg/L with a Tmax of 4-6h in healthy, fasting volunteers. 5-25% of a dose of fenofibrate is eliminated in the feces, while 60-88% is eliminated in the urine. 70-75% of the dose recovered in the urine is in the form of fenofibryl glucuronide and 16% as fenofibric acid. The volume of distribution of fenofibrate is 0.89L/kg, and can be as high as 60L. The oral clearance of fenofibrate is 1.1L/h in young adults and 1.2L/h in the elderly. Upon multiple dosing of fenofibrate, fenofibric acid steady state is achieved within 9 days. Plasma concentrations of fenofibric acid at steady state are approximately double those following a single dose. Serum protein binding was approximately 99% in normal and hyperlipidemic subjects. The absolute bioavailability of fenofibrate cannot be determined as the compound is virtually insoluble in aqueous media suitable for injection. However, fenofibrate is well absorbed from the gastrointestinal tract. Following oral administration in healthy volunteers, approximately 60% of a single dose of radiolabelled fenofibrate appeared in urine, primarily as fenofibric acid and its glucuronate conjugate, and 25% was excreted in the feces. Peak plasma levels of fenofibric acid occur within 6 to 8 hours after administration. After absorption, fenofibrate is mainly excreted in the urine in the form of metabolites, primarily fenofibric acid and fenofibric acid glucuronide. After administration of radiolabelled fenofibrate, approximately 60% of the dose appeared in the urine and 25% was excreted in the feces. The metabolism and disposition of orally administered single doses of (14)C fenofibrate (isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2- methylpropionate) have been studied in rat, guinea pig, and dog. In rats, the urinary excretion of (14)C in 5 days varied from 11 to 51% of the dose and was markedly dependent upon the dose form given. The interpretation of these data in terms of factors affecting the absorption of fenofibrate from the gut is complicated by the enterohepatic recirculation of metabolites. The tissue distribution of (14)C after oral administration of an ethanolic solution of fenofibrate has been studied in the rat. The only tissues in which the concentration of (14)C exceeded that in the blood were the organs of absorption and elimination, the gut, liver, and kidneys. Guinea pigs excreted 53% of the dose in the urine in 5 days, with a further 34% in the feces, while in dogs the corresponding figures were 9% and 81%, respectively. In all three species, all the urinary metabolites were products of ester hydrolysis, and the principal excretion product was "reduced fenofibric acid" which arose by subsequent carbonyl reduction. Glucuronidation of fenofibric acid and "reduced fenofibric acid" was a very minor reaction in the rat and guinea pig and was not detected in the dog. In addition, polar unknown metabolite(s) were detected in all three species, but were not investigated further. The results are discussed in terms of the comparative disposition of fenofibrate and other hypolipidemic agents and the contribution of these findings to the safety assessment of such drugs. Metabolism / Metabolites Fenofibrate is completely hydrolyzed by liver carboxylesterase 1 to fenofibric acid. Fenofibric acid is either glucuronidated or has its carbonyl group reduced to a benzhydrol that is then glucuronidated. Glucuronidation of fenofibrate metabolites is mediated by UGT1A9. Reduction of the carbonyl group is primarily mediated by CBR1 and minorly by AKR1C1, AKR1C2, AKR1C3, and AKR1B1. ... The metabolism of fenofibrate was investigated in cynomolgus monkeys by ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOFMS)-based metabolomics. Urine samples were collected before and after oral doses of fenofibrate. The samples were analyzed in both positive-ion and negative-ion modes by UPLC-QTOFMS, and after data deconvolution, the resulting data matrices were subjected to multivariate data analysis. Pattern recognition was performed on the retention time, mass/charge ratio, and other metabolite-related variables. Synthesized or purchased authentic compounds were used for metabolite identification and structure elucidation by liquid chromatography tandem mass spectrometry. Several metabolites were identified, including fenofibric acid, reduced fenofibric acid, fenofibric acid ester glucuronide, reduced fenofibric acid ester glucuronide, and compound X. Another two metabolites (compound B and compound AR), not previously reported in other species, were characterized in cynomolgus monkeys. More importantly, previously unknown metabolites, fenofibric acid taurine conjugate and reduced fenofibric acid taurine conjugate were identified, revealing a previously unrecognized conjugation pathway for fenofibrate. Fenofibrate has been widely used for the treatment of dyslipidemia with a long history. Species differences of its metabolism were reported, but its metabolites in rodent have not been fully investigated. Urine and plasma samples were collected before and after oral dosages of fenofibrate in Sprague-Dawley rats. Urine samples were subjected to ultra-performance liquid chromatography-electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS) analysis, and projection to latent structures discriminant analysis was used for the identification of metabolites. New metabolites in urine and plasma were also studied by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The metabolism pathway was studied in rat hepatocytes. Synthesized and purchased authentic compounds were used for metabolite identification by LC-MS/MS. Five ever-reported metabolites were identified and another four new ones were found. Among these new metabolites, fenofibric acid taurine and reduced fenofibric acid taurine indicate new phase II conjugation pathway of fenofibrate. Following oral administration, fenofibrate is rapidly hydrolyzed by esterases to the active metabolite, fenofibric acid; no unchanged fenofibrate is detected in plasma. Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine. In vivo metabolism data indicate that neither fenofibrate nor fenofibric acid undergo oxidative metabolism (e.g., cytochrome P450) to a significant extent. The metabolism and disposition of orally administered single doses of (14)C fenofibrate (isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2- methylpropionate) have been studied in rat, guinea pig, and dog. In rats, the urinary excretion of (14)C in 5 days varied from 11 to 51% of the dose and was markedly dependent upon the dose form given. The interpretation of these data in terms of factors affecting the absorption of fenofibrate from the gut is complicated by the enterohepatic recirculation of metabolites. The tissue distribution of (14)C after oral administration of an ethanolic solution of fenofibrate has been studied in the rat. The only tissues in which the concentration of (14)C exceeded that in the blood were the organs of absorption and elimination, the gut, liver, and kidneys. Guinea pigs excreted 53% of the dose in the urine in 5 days, with a further 34% in the feces, while in dogs the corresponding figures were 9% and 81%, respectively. In all three species, all the urinary metabolites were products of ester hydrolysis, and the principal excretion product was "reduced fenofibric acid" which arose by subsequent carbonyl reduction. Glucuronidation of fenofibric acid and "reduced fenofibric acid" was a very minor reaction in the rat and guinea pig and was not detected in the dog. In addition, polar unknown metabolite(s) were detected in all three species, but were not investigated further. The results are discussed in terms of the comparative disposition of fenofibrate and other hypolipidemic agents and the contribution of these findings to the safety assessment of such drugs. Route of Elimination: Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. Following oral administration in healthy volunteers, approximately 60% of a single dose of radiolabelled fenofibrate appeared in urine, primarily as fenofibric acid and its glucuronate conjugate and 25% was excreted in the feces. Half Life: 20 hours Biological Half-Life Fenofibric acid, the active metabolite of fenofibrate, has a half life of 23 hours. Fenofibrate has a half life of 19-27 hours in healthy subjects and up to 143 hours in patients with renal failure. Fenofibric acid is eliminated with a half-life of 20 hours, allowing once daily administration in a clinical setting. /Fenofibric acid/ |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Fenofibrate exerts its therapeutic effects through activation of peroxisome proliferator activated receptor a (PPARa). This increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of apoprotein C-III. The resulting fall in triglycerides produces an alteration in the size and composition of LDL from small, dense particles, to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Hepatotoxicity Mild, transient serum aminotransferase elevations develop in up to 20% of patients receiving fenofibrate, but values above 3 times normal in only 3% to 5%. These abnormalities are usually asymptomatic and transient, resolving even with continuation of fenofibrate, but they occasionally may require drug discontinuation. Monitoring of aminotransferase levels is recommended for patients receiving fenofibrate and discontinuation if enzymes persist above 3 times the upper limit of normal (ULN). There have also been multiple reports of clinically apparent liver injury in patients on fenofibrate. Onset of injury is variable; cases resembling acute hepatitis usually arise within a few weeks or months of starting therapy (Case 2), whereas cases resembling chronic hepatitis and cirrhosis typically arise after more than 6 months or even years of treatment (Case 1). The pattern of serum enzyme elevations is typically hepatocellular, but both mixed and cholestatic patterns have also been described. Some instances of acute injury with a short latency (2 to 8 weeks) are associated with fever, rash and eosinophilia, suggesting immunoallergic hepatitis. Cases with a longer latency typically present with nonspecific symptoms of weakness and fatigue, have autoimmune features with hyperglobulinemia, smooth muscle or antinuclear antibody, and a chronic hepatitis-like clinical and histological picture that is sometimes prolonged and associated with significant fibrosis or cirrhosis. Likelihood score: B (very likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No relevant published information exists on the use of fenofibrate during breastfeeding. Because of a concern with disruption of infant lipid metabolism, fenofibrate is best avoided during breastfeeding. An alternate drug is preferred, especially while nursing a newborn or preterm infant. The manufacturer recommends that breastfeeding be avoided during fenofibrate therapy and for 5 days after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Fenofibrate is 99% protein bound in serum, primarily to albumin. Toxicity Data LD50=1600 mg/kg (Oral, in mice) Interactions Caution should be exercised when anticoagulants are given in conjunction with Tricor because of the potentiation of coumarin-type anticoagulants in prolonging the prothrombin time/INR. The dosage of the anticoagulant should be reduced to maintain the prothrombin time/INR at the desired level to prevent bleeding complications. Frequent prothrombin time/INR determinations are advisable until it has been definitely determined that the prothrombin time/INR has stabilized. Increased risk of adverse musculoskeletal effects (i.e., increased CK, myoglobinuria, rhabdomyolysis). Avoid concomitant use unless potential benefit outweighs risk. Pharmacokinetic interaction reported following concomitant use with atorvastatin (decreased area under the plasma concentration-time curve [AUC] of atorvastatin) or pravastatin (increased peak plasma concentration and AUC of pravastatin). Increased risk of cyclosporine-induced nephrotoxicity (i.e., deterioration in renal function). Use with caution. Potential pharmacokinetic interaction (decreased absorption of fenofibrate). Fenofibrate should be administered 1 hour before or 4-6 hours after a bile acid sequestrant. For more Interactions (Complete) data for Fenofibrate (7 total), please visit the HSDB record page. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Fenofibrate is used as an adjunct to dietary therapy to decrease elevated serum total and LDL-cholesterol, triglyceride, and apo B concentrations, and to increase HDL-cholesterol concentrations in the management of primary hypercholesterolemia and mixed dyslipidemia, including heterozygous familial hypercholesterolemia and other causes of hypercholesterolemia. Fenofibrate also is used as an adjunct to dietary therapy in the management of patients with elevated serum triglyceride concentrations. Efficacy of the drug in reducing the risk of pancreatitis in patients with marked elevations in triglyceride concentrations (i.e., greater than 2000 mg/dL) has not been established. Fenofibrate is not indicated for use in patients with type I hyperlipoproteinemia who have elevated triglyceride and chylomicron concentrations but normal VLDL-cholesterol concentrations. /EXPL THER/ Inflammation is implicated in chronic heart failure. In this study, the potential inhibitory effect of peroxisome proliferator-activated receptor-alpha (PPARalpha) activator fenofibrate on monocyte adhesion in chronic heart failure patients was investigated in vitro. ... Isolated peripheral blood mononuclear cells were collected from 36 patients (aged 65 +/- 8 years) with symptomatic chronic heart failure and from 12 healthy control subjects. The cultured human aortic endothelial cells were stimulated with or without 2 ng mL(-1) tumor necrosis factor-alpha (TNF-alpha) and the inhibitory effects of fenofibrate at 25, 50, 100 and 200 uM on endothelial mononuclear cell adhesion were tested. Furthermore, the human aortic endothelial cells were stimulated with 70% sera obtained from chronic heart failure patients and control individuals, respectively, with or without pretreatments with fenofibrate. The endothelial expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) was then confirmed by mRNA expression and Western blot. ... The increased adhesion of peripheral blood mononuclear cells to TNF-alpha-stimulated human aortic endothelial cells in chronic heart failure patients was reduced when the human aortic endothelial cells were pretreated with fenofibrate (31% inhibition, P = 0.0121). However, pretreatment of the isolated peripheral blood mononuclear cells collected from chronic heart failure patients with fenofibrate failed to suppress their adherence to TNF-alpha-stimulated human aortic endothelial cells. Furthermore, stimulation of cultured human aortic endothelial cells with chronic heart failure patient sera significantly increased VCAM-1 and ICAM-1 expression, which could also be inhibited by fenofibrate. The fenofibrate directly inhibits monocyte binding by TNF-alpha-activated human aortic endothelial cells, probably through preventing up-regulation of cell adhesion molecules by endothelial cells in response to inflammatory stimuli. This PPARalpha activator may have the potential to ameliorate vascular inflammation in patients with chronic heart failure. Drug Warnings Severe rashes requiring hospitalization and corticosteroid therapy, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported rarely with fenofibrate in clinical studies. Urticaria and rash also have been reported in approximately 1% of patients receiving fenofibrate therapy in controlled trials. Fenofibrate, like other fibric acid derivatives (e.g., gemfibrozil), may increase cholesterol excretion in bile, resulting in cholelithiasis. If gallbladder studies indicate the presence of gallstones, fenofibrate should be discontinued. Liver function tests should be performed periodically (i.e., every 3 months) during the first 12 months of therapy. If serum aminotransferase concentrations of 3 times the upper limit of normal or higher persist, fenofibrate therapy should be discontinued. Chronic active hepatitis and cholestatic hepatitis have occurred as early as several weeks and as late as several years after initiation of fenofibrate therapy; cirrhosis associated with chronic active hepatitis has been reported rarely with fenofibrate. For more Drug Warnings (Complete) data for Fenofibrate (17 total), please visit the HSDB record page. Pharmacodynamics Fenofibrate is a fibrate that activates peroxisome proliferator activated receptor alpha (PPARα) to alter lipid metabolism and treat primary hypercholesterolemia, mixed dyslipidemia, and severe hypertriglyceridemia. Fenofibrate requires once daily dosing and has a half life of 19-27 hours so its duration of action is long. Fenofibrate capsules are given at a dose of 50-150mg daily so the therapeutic index is wide. Patients should be counselled about the risk of rhabdomyolysis, myopathy, and cholelithiasis when taking fibrates. |
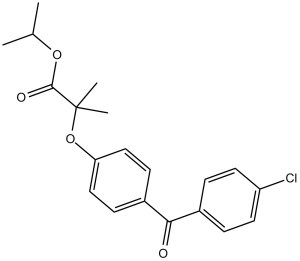
| 分子式 |
C20H21CLO4
|
|
|---|---|---|
| 分子量 |
360.83
|
|
| 精确质量 |
360.112
|
|
| CAS号 |
49562-28-9
|
|
| 相关CAS号 |
Fenofibrate (Standard);49562-28-9;Fenofibrate;49562-28-9;Fenofibrate-d6;1092484-56-4;Fenofibrate-d4;1092484-57-5
|
|
| PubChem CID |
3339
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
469.8±35.0 °C at 760 mmHg
|
|
| 熔点 |
80-81ºC
|
|
| 闪点 |
165.4±24.9 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.547
|
|
| LogP |
4.8
|
|
| tPSA |
52.6
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
458
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
YMTINGFKWWXKFG-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3
|
|
| 化学名 |
propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.93 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.93 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 33.33 mg/mL (92.37 mM) in Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7714 mL | 13.8569 mL | 27.7139 mL | |
| 5 mM | 0.5543 mL | 2.7714 mL | 5.5428 mL | |
| 10 mM | 0.2771 mL | 1.3857 mL | 2.7714 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06191133 | Not yet recruiting | Drug: Fenofibrate Procedure: Cervical Conization |
Cervical Intraepithelial Neoplasia Invasive Cervical Cancer |
Lindsay Ferguson, MD | August 1, 2024 | Phase 1 |
| NCT05514119 | Recruiting | Drug: Fenofibrate | Liver Transplant | Mayo Clinic | August 17, 2022 | Phase 2 |
| NCT05883865 | Recruiting | Drug: Larotrectinib Sulfate Procedure: Bone Scan |
Recurrent Glioma Refractory Glioma |
National Cancer Institute (NCI) |
August 23, 2017 | Phase 2 |
| 444 | Completed | Drug: Tricor (fenofibrate), 145 mg, film-coated tablet |
Hypertriglyceridemia Metabolic Syndrome |
First People's Hospital of Hangzhou | June 1, 2022 |
|