| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
L-谷氨酸可以通过在突触前水平吸收谷氨酸来影响[3H]DA的释放。即使存在 0.5 μM 河豚毒素,50 μM L-谷氨酸仍然可以诱导 [3H]DA 的释放 [1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed from the lumen of the small intestine into the enterocytes.Absorption is efficient and occurs by an active transport mechanism. /MILK/ Previous short observational studies on the free amino acid (FAA) content of human milk have shown that glutamine and glutamic acid increase in the first 4 to 6 weeks of life. Changes in human milk content of free amino acids (FAAs) was determined at colostrum, 1 month, and 3 months of lactation in 16 healthy lactating women after delivery of full-term infants. Milk was collected at the end of each feeding (hindmilk) during 24 hours. Glutamic acid and taurine were the most abundant FAAs at colostrum. Although taurine remained stable throughout lactation, glutamic acid (the prevalent FAA) and glutamine increased approximately 2.5 and 20 times, respectively, with progressing lactation representing more than 50% of total FAA at 3 months. The content of essential FAA was also stable, so the change in total FAA content was almost entirely due to the changes in glutamic acid and glutamine. Breast-fed infants are supplied with progressively increasing amounts of glutamine and glutamic acid throughout lactation. The increasing intake of glutamic acid and glutamine could benefit breast-fed infants with molecules that are likely to protect the enteral mucosa and act as neurotransmitters and as a source of nitrogen. In this report, (13)N -labeled L-glutamine and L-glutamic acid was synthesized by an enzymatic method ... . Organ distribution studies and whole body scans in mongrel dogs demonstrated low myocardial uptake of glutamine and glutamic acid and that the liver demonstrated a greater uptake of glutamine than glutamic acid or ammonia. The measurement of the intestinal metabolism of the nitrogen moiety of glutamic acid has been investigated by oral ingestion of l-[(15)N]glutamic acid and sampling of arterialized blood. Measurements have been made in six normal adults weighing an average of 72.8 kg ingesting 100 mg of l-[(15)N]glutamic acid after an overnight fast. Measurement of the enrichment of arterial glutamic acid, glutamine and alanine was by gas chromatography-mass spectrometry. Isotopic enrichment of the amino acids was followed for 150 min after the ingestion of the amino acid. Arterialized venous blood amino acid concentrations, measured by HPLC, demonstrated no significant changes during the course of the experiment. From the observed appearance of label in arterialized glutamic acid, alanine and glutamine, little luminal glutamic acid reaches the extracellular pool. The majority of the administered nitrogen label appears in the arterial alanine and glutamine components. Metabolism / Metabolites Hepatic Cortical excitability reflects a balance between excitation and inhibition. Glutamate is the main excitatory and GABA the main inhibitory neurotransmitter in the mammalian cortex. Changes in glutamate and GABA metabolism may play important roles in the control of cortical excitability. Glutamate is the metabolic precursor of GABA, which can be recycled through the tricarboxylic acid cycle to synthesize glutamate. GABA synthesis is unique among neurotransmitters, having two separate isoforms of the rate-controlling enzyme, glutamic acid decarboxylase. The need for two separate genes on two chromosomes to control GABA synthesis is unexplained. Two metabolites of GABA are present in uniquely high concentrations in the human brain. Homocarnosine and pyrrolidinone have a major impact on GABA metabolism in the human brain. Both of these GABA metabolites have anticonvulsant properties and can have a major impact on cortical excitability. /Glutamate, GABA/ The measurement of the intestinal metabolism of the nitrogen moiety of glutamic acid has been investigated by oral ingestion of l-[(15)N]glutamic acid and sampling of arterialized blood. Measurements have been made in six normal adults weighing an average of 72.8 kg ingesting 100 mg of l-[(15)N]glutamic acid after an overnight fast. Measurement of the enrichment of arterial glutamic acid, glutamine and alanine was by gas chromatography-mass spectrometry. Isotopic enrichment of the amino acids was followed for 150 min after the ingestion of the amino acid. Arterialized venous blood amino acid concentrations, measured by HPLC, demonstrated no significant changes during the course of the experiment. From the observed appearance of label in arterialized glutamic acid, alanine and glutamine, little luminal glutamic acid reaches the extracellular pool. The majority of the administered nitrogen label appears in the arterial alanine and glutamine components. Hepatic |
| 参考文献 |
[1]. Giorguieff MF, et al. Presynaptic effect of L-glutamic acid on the release of dopamine in rat striatal slices. Neurosci Lett. 1977 Oct;6(1):73-7.
|
| 其他信息 |
L-glutamic acid is an optically active form of glutamic acid having L-configuration. It has a role as a nutraceutical, a micronutrient, an Escherichia coli metabolite, a mouse metabolite, a ferroptosis inducer and a neurotransmitter. It is a glutamine family amino acid, a proteinogenic amino acid, a glutamic acid and a L-alpha-amino acid. It is a conjugate acid of a L-glutamate(1-). It is an enantiomer of a D-glutamic acid.
A peptide that is a homopolymer of glutamic acid. L-Glutamate is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). L-Glutamic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Glutamic Acid has been reported in Streptomyces akiyoshiensis, Pinus densiflora, and other organisms with data available. Glutamic Acid is a non-essential alpha-amino acid and excitatory neurotransmitter. Glutamic acid can serve as a precursor for the synthesis of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Glutamic acid (Glu), also referred to as glutamate (the anion), is one of the 20 proteinogenic amino acids. It is not among the essential amino acids. Glutamate is a key molecule in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serves as metabolic fuel or other functional roles in the body. Glutamate is the most abundant fast excitatory neurotransmitter in the mammalian nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the NMDA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, it is believed that glutamic acid is involved in cognitive functions like learning and memory in the brain. Glutamate transporters are found in neuronal and glial membranes. They rapidly remove glutamate from the extracellular space. In brain injury or disease, they can work in reverse and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via NMDA receptor channels, leading to neuronal damage and eventual cell death, and is called excitotoxicity. The mechanisms of cell death include: * Damage to mitochondria from excessively high intracellular Ca2+. * Glu/Ca2+-mediated promotion of transcription factors for pro-apoptotic genes, or downregulation of transcription factors for anti-apoptotic genes. Excitotoxicity due to glutamate occurs as part of the ischemic cascade and is associated with stroke and diseases like amyotrophic lateral sclerosis, lathyrism, and Alzheimer's disease. glutamic acid has been implicated in epileptic seizures. Microinjection of glutamic acid into neurons produces spontaneous depolarization around one second apart, and this firing pattern is similar to what is known as paroxysmal depolarizing shift in epileptic attacks. This change in the resting membrane potential at seizure foci could cause spontaneous opening of voltage activated calcium channels, leading to glutamic acid release and further depolarization. A non-essential amino acid naturally occurring in the L-form. Glutamic acid is the most common excitatory neurotransmitter in the CENTRAL NERVOUS SYSTEM. See also: Monosodium Glutamate (active moiety of); Glutamic Acid Hydrochloride (has salt form); Glatiramer Acetate (monomer of) ... View More ... Drug Indication Considered to be nature's "Brain food" by improving mental capacities; helps speed the healing of ulcers; gives a "lift" from fatigue; helps control alcoholism, schizophrenia and the craving for sugar. Mechanism of Action Glutamate activates both ionotropic and metabotropic glutamate receptors. The ionotropic ones being non-NMDA (AMPA and kainate) and NMDA receptors. Free glutamic acid cannot cross the blood-brain barrier in appreciable quantities; instead it is converted into L-glutamine, which the brain uses for fuel and protein synthesis. It is conjectured that glutamate is involved in cognitive functions like learning and memory in the brain, though excessive amounts may cause neuronal damage associated in diseases like amyotrophic lateral sclerosis, lathyrism, and Alzheimer's disease. Also, the drug phencyclidine (more commonly known as PCP) antagonizes glutamate at the NMDA receptor, causing behavior reminiscent of schizophrenia. Glutamate in action is extremely difficult to study due to its transient nature. |
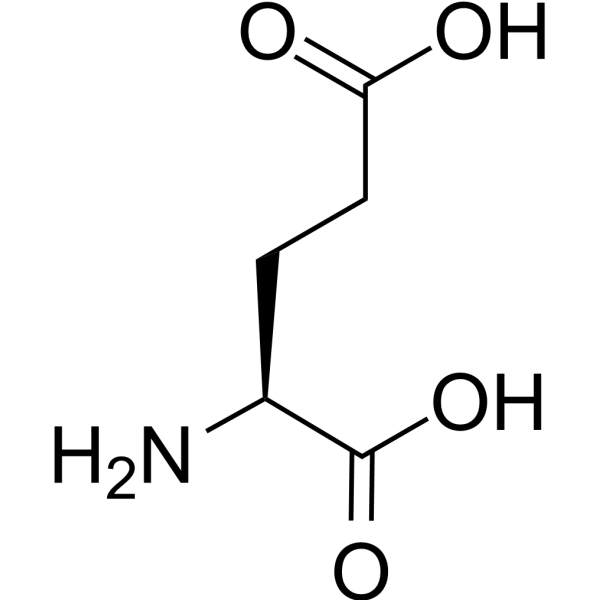
| 分子式 |
C5H9NO4
|
|---|---|
| 分子量 |
147.1293
|
| 精确质量 |
147.053
|
| CAS号 |
56-86-0
|
| 相关CAS号 |
L-Glutamic acid monosodium salt;142-47-2;L-Glutamic acid-1-13C;81201-99-2;L-Glutamic acid-15N;21160-87-2;L-Glutamic acid-13C5,15N;202468-31-3;L-Glutamic acid-13C5;55443-55-5;L-Glutamic acid-d5;2784-50-1;L-Glutamic acid-d3;203805-84-9;L-Glutamic acid-13C;115473-51-3;L-Glutamic acid-13C2;115473-56-8;L-Glutamic acid-13C5,15N,d5;1420815-74-2;L-Glutamic acid-5-13C;81202-00-8;L-Glutamic acid-15N,d5
|
| PubChem CID |
33032
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
333.8±32.0 °C at 760 mmHg
|
| 熔点 |
205 °C (dec.)(lit.)
|
| 闪点 |
155.7±25.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.522
|
| LogP |
-1.43
|
| tPSA |
100.62
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
145
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C(CC(=O)O)[C@@H](C(=O)O)N
|
| InChi Key |
WHUUTDBJXJRKMK-VKHMYHEASA-N
|
| InChi Code |
InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
|
| 化学名 |
(2S)-2-aminopentanedioic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~6.25 mg/mL (~42.48 mM)
DMSO :< 1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 9.09 mg/mL (61.78 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.7967 mL | 33.9836 mL | 67.9671 mL | |
| 5 mM | 1.3593 mL | 6.7967 mL | 13.5934 mL | |
| 10 mM | 0.6797 mL | 3.3984 mL | 6.7967 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。