Glizigen; Glycyrrhizic acid; glycyrrhizin; 1405-86-3; Glycyron; Glycyrrhizinic acid; Glycyrrhetinic acid glycoside; potenlini; Glizigen;Liquorice; Glycyrrhizin;
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
Natural triterpenoid saponinl; HMGB1; anti-tumor; anti-diabetic
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
甘草酸作为药物输送系统,具有多种抗癌相关药理活性,包括抵抗放疗和化疗引起的组织毒性、广谱抗癌能力和抗多药耐药(MDR)机制。可运输物质[1]。甘草酸显着提高钙水平并刺激分泌 GLP-1 的肠道 NCI-H716 细胞中 GLP-1 的产生。通过TGR5的激活,甘草酸可以增加GLP-1的分泌[2]。在37℃下,甘草酸可以在生理磷酸盐缓冲盐水(PBS)中产生稳定、透明的以纳米团簇为微观结构的低分子量水凝胶(LMWH)[4]。
甘草酸/Glycyrrhizic acid (GA)对转染TGR5的细胞中TGR5的直接影响[2] 与我们之前的报告一样,我们使用CHO-K1细胞转染外源性TGR5受体基因。然后,应用蛋白质印迹分析来确认实验的成功。在这些CHO-K1细胞中表达的TGR5被表征为功能性的。[2] 然后,将这些细胞用于评估Glycyrrhizic acid (GA)对TGR5受体的直接影响。在表达TGR5的细胞中,GA处理显著增强了以细胞内2-NBDG含量为指标的葡萄糖摄取(图3A)。三苯蝶啶剂量依赖性地阻断了这些细胞中GA诱导的葡萄糖摄取(图3B)。此外,GA还以剂量依赖性方式增加TGR5-CHO-K1细胞中的环AMP(cAMP)水平(图3C),并以相同的方式降低氨苯蝶啶(图3D)。然而,就2-NBDG摄取的增加(图3A)或cAMP水平的升高(图3B)而言,GA对缺乏TGR5表达的CHO-K1细胞没有影响。 甘草酸(GA)通过TGR5增强肠细胞中GLP-1的分泌[2] 具有TGR5受体的培养的肠NCI-H716细胞通常用于研究GLP-1的释放。在用GA孵育的NCI-H716细胞中也观察到GLP-1分泌(图4A)或钙内流(图4B)的显著升高。然而,在沉默NCI-H716电池中表达的TGR5基因后,GA的这两种作用都被删除了。这与我们之前的报告完全相似,该报告显示丁烯酸通过TGR5激活促进GLP-1的释放。 |
||
| 体内研究 (In Vivo) |
甘草酸升高链脲佐菌素诱导的 1 型糖尿病大鼠(STZ 治疗的大鼠)血浆 GLP-1 水平,而氨苯蝶啶足以抑制武田 G 蛋白偶联受体 5 (TGR5) 被阻断 [1]。在小鼠中,甘草酸(50 mg/kg,腹腔注射)可显着降低 TgAb、HMGB1、TNF-α、IL-6 和 IL-1β 水平 [3]。
甘草酸对1型糖尿病大鼠血糖变化的影响 [2] 在STZ诱导的糖尿病大鼠中,注射甘草酸(GA)以剂量依赖的方式减轻了高血糖(图1A)。此外,与正常大鼠(141.4±10.2 pmol/l,n=8)相比,STZ诱导的糖尿病大鼠的血浆胰岛素水平(5.35±2.11 pmol/l,n=8)显著降低。然而,最高剂量的GA未能改变这些糖尿病大鼠的血浆胰岛素水平(5.66±1.39 pmol/l,n=8)。这意味着内源性胰岛素在该动物模型中不参与GA的作用。西格列汀以有效抑制二肽基肽酶-4(DPP-4)的剂量也减轻了这些糖尿病大鼠的高血糖。西格列汀显著增强了糖尿病大鼠注射GA后高血糖的降低(图1A)。此外,无论西格列汀预处理如何,氨苯蝶啶都能剂量依赖性地抑制这些糖尿病大鼠中GA诱导的变化(图1B)。 甘草酸(GA)对糖尿病大鼠血浆GLP-1水平变化的影响 [2] 在1型糖尿病大鼠中,甘草酸(GA)诱导血浆GLP-1水平呈剂量依赖性升高(图2A)。通过以有效抑制GLP-1失活酶DPP-4的剂量用西格列汀预处理,糖尿病大鼠中甘草酸(GA)的这种作用显著增强(图2A)。此外,与血糖的变化类似,在这些糖尿病大鼠中,无论是否用西格列汀预处理,氨苯蝶啶也以剂量依赖性方式逆转了GA诱导的血浆GLP-1水平的升高(图2B)。 NaI组在8周和16周时HMGB1的mRNA表达明显高于对照组。NaI组甲状腺球蛋白抗体、HMGB1、肿瘤坏死因子α、IL-6和IL-1β的血清水平显著升高,但甘草酸(GL)注射液显著降低了这些水平。NaI+甘草酸(GL)组的甲状腺炎患病率和淋巴细胞浸润率显著降低甘草酸(GL)给药也显著降低了甲状腺中TLR2、MyD88、HMGB1和核转录因子κB的蛋白表达,减轻了甲状腺炎的严重程度。[3] 结论:HMGB1可能通过引起炎症浸润在自身免疫性甲状腺炎中起关键作用,从而增加自身免疫性甲状腺疾病的严重程度甘草酸(GL)有效地减轻了碘诱导的NOD中的甲状腺炎。H-2h4小鼠通过与抑制TLR2-HMGB1信号传导相关的分子机制[3]。 |
||
| 酶活实验 |
抗菌试验[4]
通过生长抑制效率和最小抑菌浓度(MIC)来评估GL凝胶的抗菌活性。金黄色葡萄球菌(S.aureus)和大肠杆菌(E.coli)分别被用作代表性的革兰氏阳性菌和革兰氏阴性菌。简而言之,将单个菌落接种到5 mL Luria Bertani(LB)肉汤培养基中,在37℃下以200 rpm的速度振荡培养过夜。过夜预培养后,将细菌悬浮液在LB培养基中稀释至106cfu mL-1,然后补充终浓度为0至2 mM的PBS或GL凝胶。然后将培养物在37℃下轻轻摇晃过夜。使用Synergy H1酶标仪在600nm波长下测量培养物的光密度。当没有观察到可见的细菌生长时,MIC被确定为抗菌材料的最低浓度。1遵循相同的程序实时监测用不同浓度的PBS或GL凝胶处理的细菌的生长动力学,除了在培养过程中的不同时间点记录每种培养物的OD600。特别是,将106cfu mL-1的金黄色葡萄球菌和大肠杆菌分别与终浓度为MIC的PBS或GL凝胶一起孵育。孵育2小时后,用PBS将细菌悬浮液稀释100倍,将100μL稀释的细菌铺在琼脂培养板上,在37℃下孵育过夜。 活/死荧光染色[4] 在MIC下用PBS或GL凝胶处理2小时后,通过离心收获金黄色葡萄球菌和大肠杆菌,并用0.9%NaCl洗涤3次。然后在黑暗中用SYTO 9和来自活/死染色试剂盒(L7012)的碘化丙啶(PI)对细菌进行染色30分钟。然后用LSM 700共聚焦激光扫描显微镜成像系统对细菌进行可视化。 细菌的形态学特征[4] SEM用于研究GL凝胶处理后细菌的形态。将106cfu mL-1的金黄色葡萄球菌和大肠杆菌与终浓度为MIC的PBS或GL凝胶一起孵育2小时,通过离心收集并用2.5%(w/w)戊二醛固定2小时。用PBS洗涤3次后,将细菌滴在硅片上并干燥过夜。硅片表面的细菌依次用分级乙醇水溶液(30%、50%、70%、80%、90%和100%)脱水10分钟。干燥过夜后,用金涂覆样品,并使用Phenom World Pro X扫描电子显微镜成像。 |
||
| 细胞实验 |
细胞活力测定[4]
对L929成纤维细胞进行细胞活力测定,以确定GL凝胶的体外细胞毒性。简而言之,L929细胞在添加了10%胎牛血清(FBS)的Dulbecco's Modified Eagle's Medium(DMEM)中在37℃、5%CO2的气氛中生长◦C.收获细胞,以每孔1×104个细胞的接种密度接种在96孔板中。孵育过夜后,将DMEM培养基替换为含有一定浓度的制备好的GL凝胶的新鲜培养基。孵育24小时后,用含有10%CellTiter BlueTM试剂的100μL培养基代替培养基,在37℃下孵育2小时◦C.使用Synergy H1酶标仪在550/590nm(Ex/Em)下测量荧光强度。通过与阴性对照(用PBS处理的L929细胞)进行比较来计算相对细胞存活率。 细胞成像[4] L929细胞接种在24孔板中,接种密度为每孔2×104个细胞。过夜培养后,用含有2mM GL凝胶的DMEM处理细胞,并在37℃下共培养◦用PBS洗涤处理过的细胞,并在室温下用4%(w/v)多聚甲醛固定15分钟。然后,用FITCphalloidin对细胞染色1小时,然后对细胞核酸进行DAPI染色15分钟。洗涤后,用LSM 700共聚焦激光扫描显微镜成像系统对细胞进行成像。 溶血试验[4] 通过以2000rpm离心5分钟从大鼠血液中分离红细胞(RBC)。沉淀的RBC在使用前用PBS洗涤3次。将红细胞悬浮在体积浓度为2%的PBS中,并补充终浓度为1-2mM的GL凝胶。用PBS和水处理的红细胞分别设置为阴性对照和阳性对照。在37℃下孵育2小时后,将处理过的红细胞以2000 rpm离心5分钟。使用酶标仪测量上清液在545 nm处的吸光度。溶血率使用以下方程式计算。 用于转染TGR5的CHO-K1细胞[2] 如前所述,使用成年中国仓鼠的卵巢从CHO细胞系制备CHO-K1细胞。在目前的实验中,根据我们之前的报告,使用人TGR5 cDNA的表达载体转染CHO-K1细胞。第二天,Western blot证实转染成功。然后,表达TGR5的细胞用于甘草酸(GA)处理。 细胞葡萄糖摄取的测量[2] 2-(N-(7-硝基苯-2-氧杂-1,3-二唑-4-基)氨基)-2-脱氧葡萄糖(2-NBDG)用于使用这种荧光葡萄糖类似物测定葡萄糖摄取,如我们之前的报告所述。使用荧光分光光度计测定每个细胞样品中的荧光强度。用BCA试剂盒测定蛋白质。然后,在接受甘草酸(GA)处理的细胞中定量2-NBDG的摄取。在预处理30分钟后,还将氨苯蝶啶的有效性与赋形剂治疗组进行了比较。 细胞中cAMP的测定[2] 按照我们之前的方法,用甘草酸(GA)处理细胞72小时。然后用ELISA试剂盒测定细胞内cAMP水平,类似于我们之前的法。每次测量都进行了两次。 细胞外GLP-1的测定[2] 我们使用NCI-H716细胞(每孔5×105个细胞)在37°C下用指定浓度的甘草酸(GA)处理1小时。然后,根据我们之前的方法,使用ELISA试剂盒分析细胞外GLP-1水平。对指定样品进行每次重复测量。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Glycyrrhizic acid is mainly absorbed after presystemic hydrolysis and formation of glycyrrhetinic acid. Therefore, after oral administration of a dose of 100 mg of glycyrrhizic acid, this major metabolite appears in plasma in a concentration of 200 ng/ml while glycyrrhizic acid cannot be found. The finding of a minimal amount of glycyrrhizic acid in urine suggests the existence of a partial absorption in the gastrointestinal tract. Glycyrrhizic acid presents a biphasic elimination from the central compartment with a dose-dependent second elimination phase. The majority of the administered dose is eliminated by the bile in which glycyrrhizic acid can be eliminated unchanged and undergoes enterohepatic cycling. On the other hand, the major metabolite, glycyrrhetinic acid, forms glucuronide and sulfate conjugates. These conjugates are efficiently transported into the bile and duodenum where commensal bacteria hydrolizes the conjugate for the formation of glycyrrhetinic acid and further reabsorption. This reabsorption behavior seems to be related to the activity of 3-alpha-hydroxysteroid dehydrogenase which transports very efficiently the metabolite from the plasma to the bile. About 1.1-2.5% of the administered dose of glycyrrhizic acid can be found in urine which corresponds to the minimal cycling and reabsorption of this compound. The apparent volume of distribution of glycyrrhizic acid either in the central compartment and in steady-state are in the range of 37-64 ml/kg and 59-98 ml/kg, respectively. The constant reabsorption of glycyrrhetic acid in the duodenum causes a delay in the terminal plasma clearance. The reported total body clearance of glycyrrhizic acid is reported to be in the range of 16-25 ml.kg/h. GLYCYRRHIZIN WAS ABSORBED IN RAT SMALL INTESTINE; THERE WAS NO DETECTABLE AMT OF GLYCYRRHETINIC ACID IN BLOOD AFTER BOLUS INJECTION OF GLYCYRRHIZIN INTO PORTAL VEIN; GLYCYRRHETINIC ACID WAS PRESENT IN DETECTABLE AMT IN BLOOD AFTER ORAL ADMIN. Glycyrrhizic acid (GZA) and glycyrrhetinic acid (GRA) can be determined rapidly and precisely by high-performance liquid chromatography (HPLC) in biological fluids and tissues from experimental animals and humans. From plasma and tissues, glycyrrhizic acid and glycyrrhetinic acid are extracted by organic solvents and the extracts can directly be used for HPLC. From bile or urine, extraction and determination of glycyrrhizic acid and glycyrrhetinic acid are more difficult due to interfering endogenous compounds and conjugation of glycyrrhetinic acid with glucuronides or sulfates. Extraction of glycyrrhizic acid and glycyrrhetinic acid from urine or bile can be performed by ion-pairing followed by extraction with organic solvents or by solid phase extraction. Glycyrrhetinic acid conjugates can be determined by chromatographic separation or by pretreatment with beta-glucuronidase. The pharmacokinetics of glycyrrhetinic acid and glycyrrhizic acid can be described by a biphasic elimination from the central compartment with a dose-dependent second elimination phase. Depending on the dose, the second elimination phase in humans has a half-life of 3.5 hours for glycyrrhizic acid and between 10-30 hours for glycyrrhetinic acid. The major part of both glycyrrhetinic acid or glycyrrhizic acid is eliminated by the bile. While glycyrrhizic acid can be eliminated unmetabolized and undergoes enterohepatic cycling, Glycyrrhetinic acid is conjugated to glycyrrhetinic acid glucuronide or sulfate prior to biliary excretion. Orally administered glycyrrhizic acid is almost completely hydrolyzed by intestinal bacteria and reaches the systemic circulation as glycyrrhetinic acid. Glycyrrhizic acid is currently of clinical interest for treatment of chronic hepatitis. It is also applied as a sweetener in food products and chewing tobacco. In some highly exposed subgroups of the population, serious side effects such as hypertension and electrolyte disturbances have been reported. In order to analyze the health risks of exposure to this compound, the kinetics of glycyrrhizic acid and its active metabolites were evaluated quantitatively. Glycyrrhizic acid and its metabolites are subject to complex kinetic processes, including enterohepatic cycling and presystemic metabolism. In humans, detailed information on these processes is often difficult to obtain. Therefore, a model was developed that describes the systemic and gastrointestinal tract kinetics of glycyrrhizic acid and its active metabolite glycyrrhetic acid in rats. Due to the physiologically based structure of the model, data from earlier in vitro and in vivo studies on absorption, enterohepatic cycling, and presystemic metabolism could be incorporated directly. The model demonstrates that glycyrrhizic acid and metabolites are transported efficiently from plasma to the bile, possibly by the hepatic transfer protein 3-alpha-hydroxysteroid dehydrogenase. Bacterial hydrolysis of the biliary excreted metabolites following reuptake of glycyrrhetic acid causes the observed delay in the terminal plasma clearance of glycyrrhetic acid. These mechanistic findings, derived from analysis of experimental data through physiologically based pharmacokinetic modeling, can eventually be used for a quantitative health risk assessment of human exposure to glycyrrhizic acid containing products. Copyright 2000 Academic Press. To assess the multiplicity for the biliary excretion of xenobiotic conjugates, glycyrrhizic acid (glycyrrhizin) was studied in rats after intravenous (IV) injection of 10 mg/kg glycyrrhizic acid and IV infusion of inhibitors, dibromosulfophthalein and indocyanine green. Indocyanine green did not affect the biliary excretion of glycyrrhizic acid, whereas dibromosulfophthalein reduced it significantly. The plasma level of glycyrrhizic acid was increased by dibromosulfophthalein, but not by indocyanine green. In Eisai hyperbilirubinemic rats, the biliary excretion of glycyrrhizic acid was severely impaired, resulting in an increased plasma level. The findings suggested that the biliary excretion of glycyrrhizic acid is mediated by the system shared by liquiritigenin glucuronides and dibromosulfophthalein, but not by indocyanine green, and that the system is hereditarily defective in Eisai hyperbilirubinemic rats. Metabolism / Metabolites When orally administered, glycyrrhizic acid is almost completely hydrolyzed by intestinal bacteria for the formation of glycyrrhetinic acid, which is an active metabolite and can enter systemic circulation, and two molecules of glucuronic acid. This metabolite is transported and taken in the liver for its metabolization to form glucuronide and sulfate conjugates. BOLUS INJECTION OF GLYCYRRHIZIN GIVEN RATS IN PORTAL VEIN, GAVE RISE IN BLOOD LEVEL OF SUBSTANCE WHICH APPEARS TO BE GLUCURONIC ACID CONJUGATE FORMED AS METABOLITE OF GLYCYRRHETINIC ACID. Biological Half-Life Depending on the dose, the second elimination phase in humans has a half-life of 3.5 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Licorice (Glycyrrhiza glabra) root contains glycyrrhizin (also called glycyrrhizic acid or glycyrrhizinic acid) and a mixture of the potassium and calcium salts of glycyrrhizic acid. Glycyrrhizin is metabolized to the active glycyrrhetinic acid in the intestine. Deglycyrrhizinated licorice (DGL) has had glycyrrhizin removed. Licorice is a purported galactogogue, and is included in some Asian proprietary mixtures to increase milk supply; however, no scientifically valid clinical trials support this use. In fact, licorice usually reduces serum prolactin, which might decrease milk production in the early stages of lactation. Women taking licorice have experienced elevated blood pressure. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production. Some mothers in Türkiye reportedly use licorice to improve the taste and quality of their milk. Glycyrrhizin is detectable in the breastmilk of some women taking licorice, but studies measuring glycyrrhetinic acid have not been performed. Licorice has been used safely and effectively in combination with other herbs given to infants as a tea for the short-term treatment of colic. However, two infants whose mothers had an excessive intake of an herbal tea that contained licorice had signs of anethole toxicity. Because both of these papers reported on herbal mixtures, the effect(s) of licorice alone cannot be determined. Licorice and licorice extract are "generally recognized as safe" (GRAS) as foods by the U.S. Food and Drug Administration. Long-term, excessive use of licorice can cause hypertension, hypokalemia, and disturbances of adrenal hormones, and therefore should probably be avoided during nursing. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information about dietary supplements is available elsewhere on the LactMed Web site. ◉ Effects in Breastfed Infants Two breastfed infants, aged 15 and 20 days, were admitted to the hospital for a reported lack of weight gain in the previous 7 to 10 days, caused by "difficult feeding". The parents reported restlessness and vomiting during the past day. One of the mothers also reported feeling drowsy and weak. On examination, the infants were afebrile but had hypotonia, lethargy, emesis, weak cry, poor sucking and weak responses to painful stimuli. Infant laboratory values, electrocardiograms and blood pressures were normal, and septic work-ups were negative. Both mothers had both been drinking more than 2 liters daily of an herbal tea mixture reportedly containing licorice, fennel, anise, and goat's rue to stimulate lactation. After the mothers discontinued breastfeeding and the herbal tea, the infants improved within 24 to 36 hours. Symptoms of the affected mother also resolved rapidly after discontinuing the herbal tea. After 2 days, breastfeeding was reinstituted with no further symptoms in the infants. Both infants were doing well at 6 months of age. The authors attributed the maternal and infant symptoms to anethole, which is found in both anise and fennel; however, the anethole levels were not measured in breastmilk, nor were the teas tested for their content. ◉ Effects on Lactation and Breastmilk A woman with a history of excessive licorice intake had amenorrhea, severe headaches, hypertension, hypokalemia. She had elevated serum prolactin levels that remained abnormal for one month after licorice discontinuation and normalized by 6 months after discontinuation. In a study of 25 men and 25 women, the baseline and thyrotropin-stimulated serum prolactin levels were measured to determine normal serum prolactin values. Subjects who regularly ingested licorice had lower basal and lower stimulated serum prolactin concentrations. A traditional, nonstandardized decoction of peony and licorice roots called Shaoyao-Gancao-Tang in Chinese and Shakuyaku-Kanzo-To in Japanese was studied in women with elevated serum prolactin caused by long-term (>6 months) ingestion of risperidone. Patients received either bromocriptine 5 mg daily for 4 weeks followed by 4 weeks of 22.5 grams daily of the peony-licorice decoction (equivalent to 25 mg of glycyrrhetinic acid), or the same drugs in the reverse order. Evaluation of serum prolactin found that both treatments reduced serum prolactin by 21 to 28% from baseline at 4 and 8 weeks. Forty women who complained of an insufficient milk supply at 5 days postpartum were given a combination herbal supplement as 2 capsules of Lactare (Pharma Private Ltd., Madras, India; currently available from TTK Pharma, Chennai, India) 3 times daily. Each capsule contained wild asparagus 200 mg, ashwagandha (Withania somnifera) 100 mg, fenugreek 50 mg, licorice 50 mg, and garlic 20 mg. By day 4 of therapy, no infants required supplementary feeding. Infants were weighed before and after each feeding on the fifth day of maternal therapy to determine the amount of milk ingested. On the day of the test weighing, infants' milk intake averaged 388 mL, and the fluid and caloric intake was considered adequate. This study cannot be considered as valid evidence of a galactogogue effect of these herbs because it lacks randomization, blinding, a placebo control, and maternal instruction in breastfeeding technique. Additionally, infants were breastfed only 6 to 8 times daily, which is insufficient to maximize milk supply at this stage of lactation. Women who were between 14 and 90 days postpartum and reported lactation failure were given instructions on breastfeeding technique and encouraged to exclusively breastfeed. If their infant had gained less than 15 grams in 1 week, they were randomized to receive either two tablespoonfuls of a mixture containing wild asparagus or an identical placebo for 4 weeks. In each 100 grams, the mixture contained Asparagus racemosus 15 grams, Anethum soiva 1 gram, Ipomea digitata 1 gram, Glycyrrhiza glabra 1 gram, Spinacia oleracea 2.5 grams, Cuminum cyminum 0.5 gram, and panchatrinamol 1 gram. Of the 64 women randomized, 11 did not complete the trial. Serum prolactin measurements were made before a morning nursing before treatment and after 4 weeks of treatment. Infant weight gains and the number of supplemental feedings were recorded initially and after 4 weeks of therapy. No differences were found in the changes in serum prolactin, infant weight gain or amount of supplementation between the treatment and placebo groups after 4 weeks of therapy. No side effects or changes in liver function tests occurred during the study. A study in Japan compared the use of a mixture of 13 herbs, including licorice, to ergonovine for their effects on lactation and serum prolactin in postpartum women. The herbal mixture, called Xiong-gui-tiao-xue-yin, was given in a randomized fashion to 41 women in a dose of 2 grams of a dried aqueous extract 3 times daily. A comparable group of 41 women were randomized to receive methylergonovine 0.375 mg daily. Therapy was started on the day of delivery, but the duration of therapy was not specified. Plasma oxytocin and prolactin were measured on days 1 and 6; milk volumes were measured daily, although the method of measuring milk volume was not specified. Serum prolactin was higher on days 1 and 6 in the women who received the herbals; plasma oxytocin was lower on day 1 in the women who received the herbal, but not different on day 6. Milk volumes were greater on days 4, 5, and 6 in women who received the herbal mixture. This study has serious flaws that make its interpretation impossible. First, milk volume measurement is subject to considerable variability depending on the measurement method used, but the method was not specified. Second, methylergonovine has caused decreases in serum prolactin and milk production in some studies. Because of the lack of a placebo group, the differences found could be a negative effect of methylergonovine rather than a positive effect of the herbal preparation. Because this study used a multi-ingredient combination product in which licorice was only one component, the results might be different from studies in which licorice was used alone. In an uncontrolled, non-blinded multicenter study in India, 1132 patients who reported inadequate milk supply were give a mixture (Lactancia, Corona Remedies Pvt. Ltd.) To take in a dose of 30 grams twice daily. The product contains Asparagus racemosus (wild asparagus, shatavari), Cuminum cyminum (cumin), Glycyrrhiza glabra (licorice), Spinacia oleracea (spinach) as well as amino acids, vitamins, minerals and DHA. Most of the mothers (1049) had improved lactation and increased infant weight. However, with no placebo control group, results cannot be attributed to the product. Protein Binding Glycyrrhizic acid does not bind to any plasma proteins as it is not absorbed systemically. On the other hand, its main active metabolite, glycyrrhetinic acid presents a very large binding to serum proteins such as albumin. |
||
| 参考文献 |
|
||
| 其他信息 |
Glycyrrhizinic acid is a triterpenoid saponin that is the glucosiduronide derivative of 3beta-hydroxy-11-oxoolean-12-en-30-oic acid. It has a role as an EC 3.4.21.5 (thrombin) inhibitor and a plant metabolite. It is a glucosiduronic acid, a tricarboxylic acid, a pentacyclic triterpenoid, an enone and a triterpenoid saponin. It is a conjugate acid of a glycyrrhizinate(3-).
Glycyrrhizic acid is extracted from the root of the licorice plant; Glycyrrhiza glabra. It is a triterpene glycoside with glycyrrhetinic acid that possesses a wide range of pharmacological and biological activities. When extracted from the plant, it can be obtained in the form of ammonium glycyrrhizin and mono-ammonium glycyrrhizin. Glycyrrhizic acid has been developed in Japan and China as a hepatoprotective drug in cases of chronic hepatitis. From January 2014, glycyrrhizic acid as part of the licorice extract was approved by the FDA as an existing food sweetener. It was approved by Health Canada to be used in over-the-counter products but all the products are currently on the status canceled post marketed. Glycyrrhizic acid has been reported in Hypomontagnella monticulosa, Glycyrrhiza pallidiflora, and other organisms with data available. Glycyrrhizin is a saponin-like compound that provides the main sweet flavor for Glycyrrhiza glabra (licorice), with potential immunomodulating, anti-inflammatory, hepato- and neuro-protective, and antineoplastic activities. Glycyrrhizin modulates certain enzymes involved in inflammation and oxidative stress, and downregulates certain pro-inflammatory mediators, thereby protecting against inflammation- and reactive oxygen species (ROS)-induced damage. Glycerrhizin may also suppress the growth of susceptible tumor cells. Glycyrrhyzin is a metabolite found in or produced by Saccharomyces cerevisiae. A widely used anti-inflammatory agent isolated from the licorice root. It is metabolized to GLYCYRRHETINIC ACID, which inhibits 11-BETA-HYDROXYSTEROID DEHYDROGENASES and other enzymes involved in the metabolism of CORTICOSTEROIDS. Therefore, glycyrrhizic acid, which is the main and sweet component of licorice, has been investigated for its ability to cause hypermineralocorticoidism with sodium retention and potassium loss, edema, increased blood pressure, as well as depression of the renin-angiotensin-aldosterone system. See also: Enoxolone (has active moiety); Glycyrrhizinate Dipotassium (active moiety of); Glycyrrhiza uralensis Root (part of) ... View More ... Drug Indication Glycyrrhizic acid is widely applied in foods as a natural sweetener. As a therapeutic agent, is has been used in a vast variety of formulations as it is reported to be anti-inflammatory, anti-ulcer, anti-allergic, antioxidant, anti-tumor, anti-diabetic and hepatoprotective. Due to this properties, its indications have been: treatment of premenstrual syndrome, treatment of viral infections, anti-lipidemic and antihyperglycemic. It is also known to be used as a remedy for peptic ulcer and other stomach diseases. Mechanism of Action Glycyrrhizic acid can be found in the alpha and beta forms. The alpha form is predominant in the liver and duodenum and thus, it is thought that the anti-inflammatory liver effect of this drug are mainly due to the action of this isomer. Glycyrrhizic acid anti-inflammatory effect is generated via suppression of TNF alpha and caspase 3. It also inhibits the translocation of NFkB into the nuclei and conjugates free radicals. Some studies have shown a glycyrrhizic-driven inhibition of CD4+ T cell proliferation via JNK, ERK and PI3K/AKT. The antiviral activity of glycyrrhizic acid includes the inhibition of viral replication and immune regulation. The antiviral activity of glycyrrhizic acid seems to be of a broad spectrum and be able to cover several different viral types such as vaccinia virus, herpes simplex virus, Newcastle disease virus and vesicular stomatitis virus. The effect of glycyrrhizic acid on metabolism is thought to be related to its inhibitory activity towards 11-beta-hydroxysteroid dehydrogenase type 1 which in turn decreases the activity of hexose-6-phosphate dehydrogenase. On the other hand, some studies have shown a potential lipoprotein lipase induction in non-hepatic tissues and thus it is suggested to enhance dyslipidemic conditions. GLYCYRRHIZIC ACID & ITS DERIVATIVES SHOWED PRONOUNCED ANTIINFLAMMATORY ACTION, INHIBITED DEVELOPMENT OF HISTAMINE-, SEROTONIN-, BRADKININ-, & FORMALIN-INDUCED EDEMA, & DECR VASCULAR PERMEABILITY. Drug delivery systems have become an integral part of anticancer drugs today. Design of novel drug carriers may lead to significant enhancement in antineoplastic therapy. Glycyrrhizic acid (GL), which is the most important active ingredient extracted from the licorice root shows great potential as a carrier material in this field. Recent studies have indicated that the combination of GL and first-line drugs had better therapeutic effects on cancers. GL showed a series of anti-cancer-related pharmacological activities, such as broad-spectrum anti-cancer ability, resistance to the tissue toxicity caused by chemotherapy and radiation, drug absorption enhancing effects and anti-multidrug resistance (MDR) mechanisms, as a carrier material in drug delivery systems. This review introduced the current research progress on pharmacological mechanisms of GL and development of GL-based drug carriers in anti-cancer field to provide basis for the application prospects of GL. The design of novel GL-based drug delivery systems will bring new opportunities and challenges to anti-cancer therapy.[1] Glycyrrhizic acid (GA) is belonged to triterpenoid saponin that is contained in the root of licorice and is known to affect metabolic regulation. Recently, glucagon like peptide-1 (GLP-1) has widely been applied in diabetes therapeutics. However, the role of GLP-1 in GA-induced anti-diabetic effects is still unknown. Therefore, we are interested in understanding the association of GLP-1 with GA-induced effects. In type 1-like diabetic rats induced by streptozotocin (STZ-treated rats), GA increased the level of plasma GLP-1, which was blocked by triamterene at a dose sufficient to inhibit Takeda G-protein-coupled receptor 5 (TGR5). The direct effect of GA on TGR5 has been identified using the cultured Chinese hamster ovary cells (CHO-K1 cells) transfected TGR5 gene. Moreover, in intestinal NCI-H716 cells that secreted GLP-1, GA promoted GLP-1 secretion with a marked elevation of calcium levels. However, both effects of GA were reduced by ablation of TGR5 with siRNA in NCI-H716 cells. Therefore, we demonstrated that GA can enhance GLP-1 secretion through TGR5 activation.[2] Taken together, we demonstrated for the first time that GA could increase plasma GLP-1 level via TGR5 activation in tyep 1-like diabetic rats. Therefore, GA could have future applications in the clinic. [2] igh mobility group box-1 (HMGB1), a non-histone protein, plays an important role in autoimmune diseases. However, the significance of HMGB1 in the pathogenesis of autoimmune thyroiditis has not been reported. The purpose of this study was to explore whether HMGB1 participates in the pathogenesis of autoimmune thyroiditis, and whether glycyrrhizin (GL), a direct inhibitor of HMGB1, attenuates the severity of thyroid inflammatory infiltration in a murine model of autoimmune thyroiditis.[3] Injectable low-molecular-weight hydrogels (LMWHs) from biocompatible materials have attracted much attention in biomedical applications because they can adapt any desired sizes and cavity shapes. Searching for simple, biocompatible injectable LMWHs owning inherent antibacterial activity without complicated chemical modification remains an open question to avoid the tedious synthesis/purification process and the easy bacterial infection of hydrogels in a moist environment. In this work, glycyrrhizic acid (GL), a naturally occurring compound, was found to form a stable transparent LMWH at 37 °C in physiological phosphate buffered saline (PBS) with nanoclusters as the microstructures. Moreover, this hydrogel exhibited great injectable and moldable properties. The antibacterial study showed that the growth of Gram-positive Staphylococcus aureus (S. aureus) could be completely inhibited by GL, whereas noneffect on Gram-negative Escherichia coli (E. coli) was observed. In addition, cell viability and hemolysis assay revealed that GL had good biocompatibility and hemocompatibility to mammalian cells because of its natural origin. Our simple biocompatible injectable moldable LMWH with inherent antibacterial ability has potential in the area of biomaterials and 3D bioprinting. In summary, we used natural glycyrrhizic acid as a gelator to construct a stable LMWH at 37 °C in physiological PBS without additional chemical modification. This nanocluster-structured LMWH exhibited great injectable and moldable properties. Moreover, the growth of Gram-positive S. aureus could be completely inhibited by GL compared with Gram-negative E. coli. In addition, cell viability and hemolysis assays revealed that GL has low cytotoxicity and good hemocompatibility to mammalian cells because of its natural origin. Our work provides a simple, biocompatible injectable moldable LMWH with inherent antibacterial ability, which will be of great interest in the area of biomaterials and 3D bioprinting.[4] |
| 分子式 |
C42H62O16
|
|
|---|---|---|
| 分子量 |
822.93
|
|
| 精确质量 |
822.403
|
|
| 元素分析 |
C, 61.30; H, 7.59; O, 31.11
|
|
| CAS号 |
1405-86-3
|
|
| 相关CAS号 |
Ammonium glycyrrhizinate;53956-04-0;Dipotassium glycyrrhizinate;68797-35-3
|
|
| PubChem CID |
14982
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
971.4±65.0 °C at 760 mmHg
|
|
| 熔点 |
220ºC decomposes
|
|
| 闪点 |
288.1±27.8 °C
|
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
|
| 折射率 |
1.621
|
|
| LogP |
4.64
|
|
| tPSA |
267.04
|
|
| 氢键供体(HBD)数目 |
8
|
|
| 氢键受体(HBA)数目 |
16
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
58
|
|
| 分子复杂度/Complexity |
1730
|
|
| 定义原子立体中心数目 |
19
|
|
| SMILES |
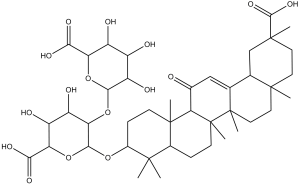
C[C@]12CC[C@](C[C@H]1C3=CC(=O)[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O[C@@H]6[C@@H]([C@H]([C@@H]([C@H](O6)C(=O)O)O)O)O[C@H]7[C@@H]([C@H]([C@@H]([C@H](O7)C(=O)O)O)O)O)C)(C)C(=O)O
|
|
| InChi Key |
LPLVUJXQOOQHMX-QWBHMCJMSA-N
|
|
| InChi Code |
InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35-,38+,39-,40-,41+,42+/m0/s1
|
|
| 化学名 |
(2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.04 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.04 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.04 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2152 mL | 6.0759 mL | 12.1517 mL | |
| 5 mM | 0.2430 mL | 1.2152 mL | 2.4303 mL | |
| 10 mM | 0.1215 mL | 0.6076 mL | 1.2152 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04028869 | Completed | Drug: Glycyrrhizic acid preparation | Autoimmune Liver Disease | Beijing Ditan Hospital | March 1, 2018 | |
| NCT04742660 | Completed | Drug: Ammonium Glycyrrhizinate | Postoperative Nausea | Konkuk University Medical Center | May 11, 2021 | Not Applicable |
| NCT05895773 | Completed | Drug: Povidone-Iodine Drug: Saline spray |
Ventilator Associated Pneumonia | Menoufia University | June 24, 2023 | Phase 2 Phase 3 |
| NCT05788705 | Not yet recruiting | Dietary Supplement: "apigenin" and "glycyrrhizin" |
Rheumatoid Arthritis | Adel A.Gomaa | July 2023 | Not Applicable |