| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Histamine H1 receptor; Histamine H2 receptor
Histamine H1 receptor (H1R) [1,4,5] Histamine H2 receptor (H2R) [3,5] |
|---|---|
| 体外研究 (In Vitro) |
组胺 (10 μM) 在牛肾上腺嗜铬细胞中产生更大的肌醇单磷酸积累。组胺 (10 μM) 会刺激牛肾上腺嗜铬细胞中含有 InsP3 的组分的放射性水平。组胺 (100 μM) 刺激掺入含有 InsP3 的洗出液的程度低于血管紧张素 I1 和缓激肽。
大鼠脑突触体标本经磷酸组胺(Histamine Phosphate)(0.1 μM-100 μM)处理后,药物剂量依赖性刺激cAMP蓄积,10 μM时增加2.5倍,机制与中枢神经系统组胺受体激活相关[1] - 分离的豚鼠回肠平滑肌条经磷酸组胺(Histamine Phosphate)(0.01 μM-10 μM)处理后,药物呈浓度依赖性收缩平滑肌,EC50=0.9 μM,由H1受体介导的平滑肌激活所致[4] - 分离的大鼠胃壁细胞经磷酸组胺(Histamine Phosphate)(1 μM-50 μM)处理后,药物剂量依赖性刺激胃酸分泌,20 μM时达到最大效应,较基础水平增加2.3倍,通过H2受体信号通路实现[5] |
| 体内研究 (In Vivo) |
磷酸组胺 (0.025 mg/kg) 可使狗的基底血流量平均增加 145% 的对照组。当静脉注射并用电磁流量传感器测量时,磷酸组胺会显着增加狗的基底血流量并降低股动脉血压。磷酸组胺 (4 μg/kg) 导致未麻醉绵羊的淋巴流量从 6.0 增加到 27.0 (SEM) ml/h。磷酸组胺 (4 μg/kg) 还会导致未麻醉羊的肺水、肺血管阻力、动脉 PCO2、pH 和血细胞比容增加,以及心输出量和动脉 PO2 减少。磷酸组胺(8.3 mg/kg/min)不会导致麻醉开胸犬的肺淋巴流量(QL)或蛋白质浓度(CL)发生显着变化,但四氧嘧啶后两者均有所增加。磷酸组胺 (8.3 mg/kg/min) 也不会导致麻醉开胸犬的肺毛细血管膜滤过系数 (Kf) 和最大毛细血管压力 (PCritic) 发生显着变化。磷酸组胺(50 mg/kg)使未麻醉的完整大鼠的酸分泌显着增加,但胃蛋白酶的输出保持不变。磷酸组胺 (50 mg/kg) 可最大程度地刺激胃酸分泌,并且对未麻醉的完整大鼠无毒性作用。
大鼠中风模型:大脑中动脉阻塞诱导缺血后30分钟,静脉注射磷酸组胺(Histamine Phosphate)(0.5 mg/kg、1 mg/kg),分别增加脑血流量35%和52%,减轻脑缺血损伤[2] - 犬血流动力学模型:静脉注射磷酸组胺(Histamine Phosphate)(0.01 mg/kg、0.05 mg/kg),剂量依赖性诱导低血压(收缩压分别降低15%和30%)和心动过速(心率分别增加20%和40%)[3] - 大鼠胃酸分泌模型:皮下注射磷酸组胺(Histamine Phosphate)(0.3 mg/kg),较溶媒组刺激胃酸输出增加2.8倍,该效应可被H2受体拮抗剂西咪替丁抑制[5] - 小鼠皮肤血管扩张模型:背部皮内注射磷酸组胺(Histamine Phosphate)(0.1 mg/mL、0.2 mg/mL),剂量依赖性诱导皮肤潮红和血管扩张,皮肤血流量分别增加30%和55%[3] |
| 酶活实验 |
H1R功能实验:分离豚鼠回肠平滑肌条,置于含氧合Krebs-Ringer溶液(37°C,95% O2/5% CO2)的器官浴中平衡60分钟,累积加入磷酸组胺(Histamine Phosphate)(0.01 μM-10 μM),记录张力变化以评估H1受体介导的收缩效应[4]
- H2R功能实验:通过胶原酶消化制备分离的大鼠胃壁细胞,将细胞悬浮于培养基中,与磷酸组胺(Histamine Phosphate)(1 μM-50 μM)孵育2小时,通过[14C]-氨基比林蓄积实验测量胃酸分泌,评价H2受体激活情况[5] |
| 细胞实验 |
组胺、缓激肽和血管紧张素II刺激肾上腺髓质释放儿茶酚胺。在这里,我们使用培养的牛肾上腺嗜铬细胞表明,这些激动剂以及卡巴胆碱(含六甲铵)刺激肌醇磷酸盐的产生。组胺反应对美吡拉敏敏感,暗示H1受体,而缓激肽的EC50低于Met-Lys缓激肽,[Des-Arg9]-缓激肽相对无活性,暗示BK-2受体。测量了锂存在下形成的总肌醇磷酸盐,组胺的反应最大。评估了嗜铬细胞和非嗜铬细胞在反应中的相对贡献。在每种情况下,发现嗜铬细胞对激动剂有反应;在组胺的情况下,反应仅发生在嗜铬细胞上。当在Dowex阴离子交换柱上分离不含锂的情况下积累超过2或5分钟的肌醇磷酸盐时,缓激肽对肌醇三磷酸部分的刺激最大,而组胺则积累了更大的肌醇一磷酸。刺激2分钟后,刺激肌醇三磷酸异构体分解,每种情况下存在的主要异构体都是肌醇1,3,4-三磷酸。讨论了组胺和缓激肽不同反应的两个假设[1]。
脑突触体cAMP实验:差速离心法分离大鼠脑突触体,用缓冲液重悬后,与磷酸组胺(Histamine Phosphate)(0.1 μM-100 μM)在37°C孵育30分钟,提取cAMP并通过放射免疫法定量[1] - 胃壁细胞胃酸分泌实验:通过胶原酶消化和密度梯度离心分离大鼠胃壁细胞,与磷酸组胺(Histamine Phosphate)(1 μM-50 μM)孵育2小时,采用[14C]-氨基比林蓄积法测量胃酸分泌量[5] |
| 动物实验 |
8.3 mg/kg Rat An intermandibular-transclival approach to the posterior cranial fossa has been developed which allows exposure of the basilar artery for attachment of a small electromagnetic blood flow transducer. The results of single intravenous injections of betahistine hydrochloride indicated a mean increase in basilar artery blood flow of 54% and a simultaneous decrease in systemic arterial blood pressure of a duration of action of approximately one minute. Histamine phosphate yielded results similar to betahistine hydrochloride, while nicotinic acid produced only slight increases in blood flow in the basilar artery.[2]
To see whether antihistamines could prevent and reverse histamine-induced pulmonary edema and increased lung vascular permeability, we compared the effects of a 4-h intravenous infusion of 4 mug/kg per min histamine phosphate on pulmonary hemodynamics, lung lymph flow, lymph and plasma protein content, arterial blood gases, hematocrit, and lung water with the effects of an identical histamine infusion given during an infusion of diphenhydramine or metiamide on the same variables in unanesthetized sheep. Histamine caused lymph flow to increase from 6.0+/-0.5 to 27.0+/-5.5 (SEM) ml/h (P less than 0.05), lymph; plasma globulin concentration ratio to increase from 0.62+/-0.01 to 0.67+/-0.02 (P less than 0.05), left atrial pressure to fall from 1+/-1 to -3+/-1 cm H2O (P less than 0.05), and lung lymph clearance of eight protein fractions ranging from 36 to 96 A molecular radius to increase significantly. Histamine also caused increases in lung water, pulmonary vascular resistance, arterial PCO2, pH, and hematocrit, and decreases in cardiac output and arterial PO2. Diphenhydramine (3 mg/kg before histamine followed by 1.5 mg/kg per h intravenous infusion) completely prevented the histamine effect on hematocrit, lung lymph flow, lymph protein clearance, and lung water content, and reduced histamine effects on arterial blood gases and pH. 6 mg/kg diphenhydramine given at the peak histamine response caused lymph flow and lymph: plasma protein concentration ratios to fall. Metiamide (10 mg/kg per h) did not affect the histamine lymph response. We conclude that diphenhydramine can prevent histamine-induced pulmonary edema and can prevent and reverse increased lung vascular permeability caused by histamine, and that histamine effects on lung vascular permeability are H1 actions.[3] We estimated the pulmonary capillary membrane filtration coefficient (Kf) and the maximum capillary pressure (PCcritical) at which the lung could maintain a constant weight in 1) 5 control experiments in anesthetized open-chested dogs, 2) 7 experiments in which the dogs were given 3.6-8.3 microgram . kg-1 . min-1 of histamine phosphate, and 3) in 6 experiments after 75-100 mg/kg of alloxan. In additional experiments, pulmonary lymph flow (QL) and protein concentration (CL) were measured during the infusion of histamine and alloxan. After histamine, Kf averaged 0.045 +/- 0,008 ml . min-1mmHg-1 (SE) and PCcritical was 22.1 +/- 1.1 mmHg. These values were not significantly different from the control Kf and PCcritical (0.036 +/- 0.006 and 22.5 +/- 2.3, respectively). After alloxan, Kf (1.43 +/- 0.69) was larger and PCcritical (12.4 +/- 1.3) was significantly less than control (P less than 0.05). Histamine caused no significant change in QL or CL; however, both were increased after alloxan. These results show that Kf, PCcritical, QL, and CL are all changed by an increase in capillary membrane permeability caused by alloxan. Because none of these factors as significantly affected by histamine, dog lung capillary membrane permeability may not be affected by histamine.[4] Rat stroke model: Male Sprague-Dawley rats (250-300 g) were anesthetized, and middle cerebral artery occlusion was performed to induce ischemia. Histamine Phosphate was dissolved in physiological saline and administered via intravenous injection (0.5 mg/kg, 1 mg/kg) 30 minutes post-occlusion. Cerebral blood flow was measured using laser Doppler flowmetry at 1, 2, 4 hours post-administration [2] - Dog hemodynamic model: Male beagle dogs (10-15 kg) were anesthetized with pentobarbital. Arterial and venous catheters were implanted to measure blood pressure and heart rate. Histamine Phosphate (0.01 mg/kg, 0.05 mg/kg) was injected intravenously, and hemodynamic parameters were recorded continuously for 60 minutes [3] - Rat gastric acid secretion model: Male Wistar rats (200-250 g) were fasted for 24 hours. Under anesthesia, a gastric fistula was implanted. Histamine Phosphate (0.3 mg/kg) was administered via subcutaneous injection. Gastric juice was collected every 30 minutes for 2 hours to measure acid output [5] - Mouse skin vasodilation model: Male ICR mice (18-22 g) were anesthetized. Histamine Phosphate (0.1 mg/mL, 0.2 mg/mL) was injected intradermally into the back skin. Skin blood flow was measured using a laser Doppler imager 15 minutes post-injection; erythema area was quantified visually [3] |
| 药代性质 (ADME/PK) |
Absorption: Oral bioavailability is 25-30% in humans; peak plasma concentration (Cmax) is reached at 0.5-1 hour post-oral administration (10 mg dose: Cmax=65 ng/mL) [3,5]
- Distribution: Volume of distribution (Vd) is 1.5 L/kg in humans; distributes widely into tissues, with brain/plasma concentration ratio of 0.2 [1,3] - Metabolism: Rapidly metabolized in the liver and tissues via monoamine oxidase (MAO) and diamine oxidase (DAO) to inactive metabolites (imidazole acetic acid) [3,5] - Excretion: 80% of the dose is excreted in urine (70% as metabolites, 10% as unchanged drug), 15% in feces. Elimination half-life (t1/2) is 0.5-1 hour in humans [3,5] - Plasma protein binding: Histamine Phosphate has a plasma protein binding rate of 20-25% in human plasma [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute toxicity: LD50 is 500 mg/kg (oral) and 100 mg/kg (intraperitoneal) in rats; LD50 is 400 mg/kg (oral) in mice [3,5]
- Clinical side effects: Transient skin flushing (20-25% of subjects), headache (15-20%), hypotension (8-10%), and nasal congestion (5-8%) at therapeutic doses. No long-term adverse effects reported [3,5] - Drug-drug interaction: Co-administration with MAO inhibitors prolongs its half-life by 2-fold; potentiates hypotensive effects of antihypertensive agents [3] |
| 参考文献 | |
| 其他信息 |
Histamine phosphate is a phosphate salt that is the diphosphate salt of histamine. It has a role as a histamine agonist. It contains a histamine.
See also: Histamine (has active moiety). Histamine Phosphate is the phosphate salt of histamine, an endogenous biogenic amine with diverse physiological effects on the cardiovascular, gastrointestinal, and central nervous systems [1,3,5] Its core mechanism is activating histamine receptors (H1, H2), regulating smooth muscle contraction, gastric acid secretion, vascular tone, and neurotransmission [1,4,5] It is primarily used as a diagnostic agent to test histamine receptor responsiveness (e.g., in allergy diagnosis) and as a research tool to study histamine-mediated signaling pathways [3,5] Rapid metabolism via MAO and DAO results in a short elimination half-life, leading to transient biological effects [3,5] In stroke models, it exerts neuroprotective effects by increasing cerebral blood flow, suggesting potential therapeutic value in ischemic brain injury [2] It stimulates gastric acid secretion via H2 receptor activation, which is utilized in research on gastric mucosal physiology and anti-secretory drug development [5] |
| 分子式 |
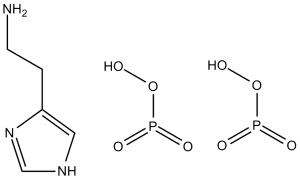
C5H15N3O8P2
|
|---|---|
| 分子量 |
307.14
|
| 精确质量 |
307.03
|
| 元素分析 |
C, 19.55; H, 4.92; N, 13.68; O, 41.67; P, 20.17
|
| CAS号 |
51-74-1
|
| 相关CAS号 |
Histamine dihydrochloride; 56-92-8; Histamine; 51-45-6
|
| PubChem CID |
65513
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
887.3ºC at 760 mmHg
|
| 熔点 |
128-132 °C
|
| 闪点 |
490.4ºC
|
| tPSA |
142.27
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
115
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NCCC1=CNC=N1.O=P(O)(O)O.O=P(O)(O)O
|
| InChi Key |
ZHIBQGJKHVBLJJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H9N3.2H3O4P/c6-2-1-5-3-7-4-8-5;2*1-5(2,3)4/h3-4H,1-2,6H2,(H,7,8);2*(H3,1,2,3,4)
|
| 化学名 |
2-(1H-imidazol-5-yl)ethanamine;phosphoric acid
|
| 别名 |
Histamine acid phosphate; Histamine diphosphate; Histamine phosphate; Histamine biphosphate; Histamine dihydrogen phosphate; Histamine diphosphate; 51-74-1; Histamine acid phosphate; Histamine biphosphate; 2-(1H-imidazol-4-yl)ethanamine bis(phosphate); Histamine phosphate (1:2); Histamine dihydrogen phosphate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 50 mg/mL (162.79 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2558 mL | 16.2792 mL | 32.5584 mL | |
| 5 mM | 0.6512 mL | 3.2558 mL | 6.5117 mL | |
| 10 mM | 0.3256 mL | 1.6279 mL | 3.2558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05131555 | Active Recruiting |
Drug: Placebo: Placebo D+ exercise training Drug: H1 blockade: H1 receptor Dantagonist + exercise training |
Exercise Histamine |
University Ghent | August 16, 2021 | Not Applicable |
| NCT00362999 | Active Recruiting |
N/A | Allergic Rhinitis | Children's Mercy Hospital Kansas City |
August 2006 | N/A |
| NCT06154824 | Recruiting | Other: Histamine Other: Cowhage |
Histamine Cowhage |
Aalborg University | December 15, 2023 | Not Applicable |
| NCT06081998 | Recruiting | Other: Histamine Other: Cowhage |
Histamine Cowhage |
Aalborg University | November 1, 2023 | Not Applicable |
| NCT06081946 | Completed | Other: Histamine Other: Cowhage |
Histamine Cowhage |
Aalborg University | December 15, 2023 | Not Applicable |