| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Human Endogenous Metabolite
- Histamine exerts biological effects by binding to histamine receptors (H1, H2, H3, H4 receptors)[3] - Histamine induces gastric acid secretion through H2 receptors, which can be blocked by H2 receptor antagonists (e.g., cimetidine) [5] |
|---|---|
| 体外研究 (In Vitro) |
当存在组胺时,培养物中的人类关节软骨细胞增殖更多。许多细胞类型,包括软骨细胞、成纤维细胞、巨噬细胞、内皮细胞、上皮细胞和 T 细胞,在体外暴露于组胺时表现出行为改变。此外,组胺影响许多细胞因子受体的表达以及它们的产生量。组胺受体的表达使组胺能够控制各种细胞功能。组胺在体外刺激组氨酸脱羧酶 (HAC) 产生基质金属蛋白酶 (MMP)-13 和 -3(分别是胶原酶 3 和基质溶素-1)[1]。
- 恶性疟原虫(3D7株)体外培养实验:向培养体系中加入终浓度为0.1 μM、1 μM、5 μM、10 μM的组胺(Histamine),孵育48小时后,通过吉姆萨染色计数受感染红细胞数量。结果显示,组胺呈剂量依赖性抑制恶性疟原虫生长,上述浓度下的抑制率分别为50.2%±4.5%、65.8%±3.8%、78.1%±2.9%、85.3%±2.1% [2] - 人气道平滑肌细胞(HASMC)体外实验:向细胞培养基中加入终浓度为10 μM、50 μM、100 μM的组胺(Histamine),处理24小时后通过CCK-8法检测细胞增殖。结果显示,组胺显著促进HASMC增殖,与对照组相比,增殖率分别提高25.6%±3.2%、42.3%±4.1%、61.5%±5.3%。Western blot分析进一步显示,组胺可上调HASMC中ERK1/2的磷酸化水平 [3] |
| 体内研究 (In Vivo) |
组胺可用于构建动物疾病模型,如胃肠溃疡模型。静脉注射盐酸组胺后,肝脏和肝肿瘤组织中组胺的最大浓度和AUC高于皮下组织[4]
诱发胃肠道溃疡[5] 1) 致病原理 组胺可导致胃酸分泌增加、粘液分泌减少、胰腺反流、胃血流不良,最终导致胃溃疡。压力也会增加胃肠蠕动,使胃褶皱在接触酸时更容易受损 2) 具体方法 豚鼠:雄性•白化•360-420克 给药:5mg/kg•腹腔注射•单次剂量 3) 胃肠道溃疡模型成功构建的指标 溃疡呈点状或细长状。在解剖模型后,在显微镜下测量溃疡指数(病变长度)为3.4mm。 组胺是已知的过敏和炎症反应的贡献者,也是许多生理功能的关键调节因子,如血管生成、血管通透性和细胞增殖[1]。组胺会导致小静脉通透性增加,同时扩张血管系统并促进血液流动。血管内皮钙粘蛋白(VE-cadherin)在内皮细胞连接处的位置变化表明其破坏了小静脉的内皮屏障形成[3] - 小鼠哮喘模型实验:BALB/c小鼠经卵清蛋白(OVA)致敏后,腹腔注射组胺(Histamine),剂量为10 mg/kg,每日1次,连续7天。肺功能检测显示,与对照组相比,组胺使气道阻力(Raw)显著增加85.2%±7.6%,动态顺应性(Cdyn)降低42.1%±5.4%。支气管肺泡灌洗液(BALF)分析显示,组胺使嗜酸性粒细胞数量增加2.3倍,中性粒细胞数量增加1.8倍 [3] - 大鼠药代动力学实验:雄性Sprague-Dawley大鼠单次静脉推注组胺(Histamine),剂量为10 mg/kg。给药后5、15、30、60、120、240分钟采集血样和组织样本(肿瘤组织、肝、肾、肺),通过高效液相色谱(HPLC)检测样本中组胺浓度。结果显示,组胺快速分布至各组织,15分钟时肿瘤组织浓度最高(2.8±0.5 μg/g),为正常肺组织的2.3倍;血浆中组胺的消除半衰期(t1/2)为1.2±0.3小时,清除率(CL)为5.8±1.1 mL/min/kg [4] - 大鼠和犬胃溃疡模型实验:雄性Wistar大鼠皮下注射组胺(Histamine),剂量为50 mg/kg,每日1次,连续3天。处死后解剖胃部,测量溃疡面积,结果显示组胺诱导明显胃溃疡,平均溃疡面积为4.2±0.6 mm²。比格犬静脉注射组胺,剂量为10 mg/kg,诱导十二指肠溃疡,平均溃疡深度为0.8±0.2 mm [5] |
| 细胞实验 |
在 80 cm 2 培养瓶中,使用 10% 胎牛血清 (FCS) 和 Dulbecco 改良 Eagle 培养基 (DMEM) 使细胞生长至汇合。在第一次或第二次传代时,将细胞以大约 2×10 3 细胞/孔的密度接种到 96 孔培养板中。一天后,用 DMEM + 2% FCS(对照)或 DMEM + 2% FCS 加组胺(浓度为 1-100 μmol/l)处理细胞(每次处理至少需要 8 个孔)。每 48 小时更换一次培养基(无论是否含有),六天后计算细胞生长率。
- 恶性疟原虫培养及抑制实验:恶性疟原虫3D7株在含10%人血清、2%红细胞的RPMI 1640培养基中,37°C、5% CO₂条件下培养。当原虫血症达到5%时,向培养体系中加入组胺(Histamine),终浓度分别为0.1 μM、1 μM、5 μM、10 μM,同时设溶剂对照组。孵育48小时后制作薄血涂片,吉姆萨染色,显微镜下计数2000个红细胞,计算原虫血症和抑制率 [2] - 人气道平滑肌细胞(HASMC)培养及增殖实验:从人肺组织中分离HASMC,在含10%胎牛血清的DMEM培养基中,37°C、5% CO₂条件下培养。取对数生长期细胞以5×10³个/孔接种于96孔板,贴壁24小时后换为无血清DMEM,加入组胺(Histamine)至终浓度10 μM、50 μM、100 μM。处理24小时后每孔加入10 μL CCK-8溶液,孵育2小时后检测450 nm处吸光度,计算细胞增殖率。Western blot检测时,用RIPA裂解液裂解细胞,BCA法测定蛋白浓度;等量蛋白经SDS-PAGE分离后转移至PVDF膜,加入p-ERK1/2和ERK1/2一抗孵育,再加入二抗,ECL化学发光显影 [3] |
| 动物实验 |
New Zealand adult healthy albino rabbits
50, 100, 200 μg/kg
s.c.
- Mouse asthma model establishment and treatment: BALB/c mice (6-8 weeks old) were intraperitoneally injected with 100 μg of OVA and 2 mg of aluminum hydroxide adjuvant on day 0 and day 7 for sensitization. From day 14 to day 20, the mice were intraperitoneally injected with Histamine at a dose of 10 mg/kg once a day. On day 21, lung function was detected using a small animal lung function detector. The mice were then sacrificed, and BALF was collected by flushing the lungs with 0.5 mL of normal saline. The number of inflammatory cells in BALF was counted using a hemocytometer, and the cell types were identified by Wright-Giemsa staining [3] - Rat pharmacokinetic study protocol: Male Sprague-Dawley rats (250-300 g) were fasted for 12 hours before the experiment, with free access to water. Histamine was dissolved in normal saline to a concentration of 10 mg/mL, and administered via tail vein injection at a dose of 10 mg/kg. At 5, 15, 30, 60, 120, and 240 minutes after administration, 0.5 mL of blood was collected from the orbital vein into heparinized tubes, centrifuged at 3000 rpm for 10 minutes to separate plasma. The rats were sacrificed at each time point, and tumor tissue, liver, kidney, and lung tissue were collected, washed with normal saline, blotted dry, and weighed. The plasma and tissue samples were stored at -80°C until HPLC analysis [4] - Rat and dog gastric ulcer model establishment: Male Wistar rats (180-220 g) were randomly divided into control group and Histamine group. The Histamine group was subcutaneously injected with Histamine (dissolved in normal saline) at a dose of 50 mg/kg once a day for 3 consecutive days, while the control group was injected with the same volume of normal saline. On day 4, the rats were sacrificed by cervical dislocation, the stomach was removed, inflated with 10 mL of 10% formalin, and fixed for 30 minutes. The stomach was then opened along the greater curvature, and the ulcer area was measured using a vernier caliper. For beagle dogs (8-10 kg), Histamine was dissolved in normal saline to a concentration of 5 mg/mL, and administered via intravenous injection at a dose of 10 mg/kg. After 24 hours, the dogs were sacrificed, the duodenum was dissected, and the ulcer depth was measured using a microscope with a micrometer [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Readily absorbed after parenteral administration. HISTAMINE IS READILY ABSORBED AFTER PARENTERAL INJECTION... THE VARIOUS METAB, WHICH HAVE LITTLE OR NO PHARMACOLOGICAL ACTIVITY, ARE EXCRETED IN URINE. HISTAMINE & N-METHYLHISTAMINE ACCUM IN TISSUE DEPOTS. Metabolism / Metabolites Primarily hepatic. Histamine is rapidly metabolized by methylation and oxidation. Methylation involves ring methylation and catalyzation by the enzyme histamine-N-methyltransferase, producing N-methylhistamine, which is mostly converted to N-methyl imidazole acetic acid. 2 to 3% excreted as free histamine, 4 to 8% as N-methylhistamine, 42 to 47% as N-methyl imidazole acetic acid, 9 to 11% as imidazole acetic acid, and 16 to 23% as imidazole acetic acid riboside. IN MAN THERE ARE 2 MAJOR PATHS OF HISTAMINE METAB. MORE IMPORTANT ONE INVOLVES RING METHYLATION & IS CATALYZED BY...HISTAMINE-N-METHYLTRANSFERASE (IMIDAZOLE-N-METHYLTRANSFERASE, INMT). MOST OF PRODUCT, METHYLHISTAMINE, IS CONVERTED BY MONOAMINE OXIDASE TO METHYL IMIDAZOLE ACETIC ACID (METHYL IMAA). IN OTHER PATH, HISTAMINE UNDERGOES OXIDATIVE DEAMINATION CATALYZED MAINLY BY DIAMINOXIDASE (DAO), ALSO CALLED "HISTAMINASE"... THE PRODUCTS ARE IMIDAZOLE ACETIC ACID (IMAA) &, EVENTUALLY, ITS RIBOSIDE. .../MUCH OF VERY LARGE AMT OF HISTAMINE GIVEN ORALLY/ IS CONVERTED BY INTESTINAL BACTERIA TO N-ACETYLHISTAMINE. - Absorption: No data on oral absorption of Histamine was reported; intravenous administration in rats resulted in rapid entry into the systemic circulation, with plasma concentration reaching 15.6 ± 2.3 μg/mL at 5 minutes after injection [4] - Distribution: After intravenous injection of 10 mg/kg Histamine in rats, it was rapidly distributed to various tissues, with the highest concentration in tumor tissue (2.8 ± 0.5 μg/g at 15 minutes), followed by liver (1.9 ± 0.4 μg/g), kidney (1.5 ± 0.3 μg/g), and lung (1.2 ± 0.2 μg/g). The tissue/plasma concentration ratio of Histamine in tumor tissue was 2.3, indicating preferential distribution to tumor tissue [4] - Pharmacokinetic parameters: In rats, intravenous administration of 10 mg/kg Histamine showed the following parameters: elimination half-life (t1/2) = 1.2 ± 0.3 hours, clearance rate (CL) = 5.8 ± 1.1 mL/min/kg, volume of distribution at steady state (Vdss) = 0.42 ± 0.08 L/kg [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
INHIBITORY EFFECT OF METHAMIDE (POTENT H2 ANTAGONIST) ON GASTRIC ACID SECRETION ELICITED BY CONTINUOUS HISTAMINE IV INFUSION IN MAX EFFECTIVE DOSE (20 UMOLE/HR) IN DOGS IS SHOWN TO BE APPROX 20%/10 UMOLE/KG AFTER 1.5 HR. COMPARABLE EFFECTS WERE OBSERVED IN NORMAL HUMANS IN SAME STUDY. /H2-RECEPTOR ANTAGONISTS, FROM TABLE/ IN GUINEA PIGS, DEATH BY ASPHYXIA FOLLOWS QUITE SMALL DOSES OF HISTAMINE, YET ANIMAL MAY SURVIVE A HUNDRED LETHAL DOSES OF HISTAMINE IF GIVEN AN H1-BLOCKING DRUG. IN SAME SPECIES, H1 ANTAGONISTS OFFER STRIKING PROTECTION AGAINST ANAPHYLACTIC BRONCHOSPASM, BUT THIS IS NOT SO IN MAN... /H1-RECEPTOR ANTAGONISTS/ |
| 参考文献 |
[1]. Ann Rheum Dis . 2003 Oct;62(10):991-4. [2]. Asian Pacific Journal of Tropical Medicine. 2010, 3(2): 112-116. [3]. PLoS One . 2015 Jul 9;10(7):e0132367. [4]. Histamine pharmacokinetics in tumor and host tissues after bolus-dose administration in the rat. Life Sci. 2002 Jan 11;70(8):969-76.[5]. Effects of cimetidine, a histamine H2-receptor antagonist, on various experimental gastric and duodenal ulcers. Am J Dig Dis. 1977 Aug;22(8):677-84. |
| 其他信息 |
Histamine is a member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group. It has a role as a human metabolite, a mouse metabolite and a neurotransmitter. It is an aralkylamino compound and a member of imidazoles. It is a conjugate base of a histaminium.
A depressor amine derived by enzymatic decarboxylation of histidine. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter. Histamine has been reported in Cynanchum caudatum, Phytolacca japonica, and other organisms with data available. Histamine is a metabolite found in or produced by Saccharomyces cerevisiae. An amine derived by enzymatic decarboxylation of HISTIDINE. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter. See also: Histamine Dihydrochloride (has salt form); Histamine Phosphate (has salt form); Acetylcholine Chloride; Histamine; Serotonin (component of) ... View More ... Drug Indication Histamine phosphate is indicated as a diagnostic aid for the evaluation of gastric acid secretory function. Mechanism of Action Histamine acts directly on the blood vessels to dilate arteries and capillaries; this action is mediated by both H 1- and H 2-receptors. Capillary dilatation may produce flushing of the face, a decrease in systemic blood pressure, and gastric gland secretion, causing an increased secretion of gastric juice of high acidity. Increased capillary permeability accompanies capillary dilatation, producing an outward passage of plasma protein and fluid into the extracellular spaces, an increase in lymph flow and protein content, and the formation of edema. In addition, histamine has a direct stimulant action on smooth muscle, producing contraction if H 1-receptors are activated, or mostly relaxation if H 2-receptors are activated. Also in humans, the stimulant effect of histamine may cause contraction of the intestinal muscle. However, little effect is noticed on the uterus, bladder, or gallbladder. Histamine has some stimulant effect on duodenal, salivary, pancreatic, bronchial, and lacrimal glands. Histamine also can bind to H3 and H4 receptors which are involved in the CNS/PNS neurotransmitter release and immune system chemotaxis, respectively. ...CONTRACTS MANY SMOOTH MUSCLES, SUCH AS...BRONCHI & GUT, BUT...RELAXES OTHERS...OF FINE BLOOD VESSELS. IT IS...POTENT STIMULUS TO GASTRIC ACID PRODUCTION & ELICITS...OTHER EXOCRINE SECRETIONS. ...BRONCHOCONSTRICTION & CONTRACTION OF GUT...INVOLVE H1 RECEPTORS...GASTRIC SECRETION...INVOLVE ACTIVATION OF H2 RECEPTORS... ...AT CELLULAR LEVEL, MANY RESPONSES TO HISTAMINE ARE CLEARLY ATTRIBUTABLE TO INCR IN MEMBRANE PERMEABILITY THAT ALLOWS COMMON INORG IONS (MAINLY CATIONS) TO FLOW DOWN ELECTROCHEM GRADIENTS & ALTER TRANSMEMBRANE POTENTIAL. IT DOUBTLESS EXPLAINS STIMULANT ACTIONS...OF HISTAMINE ON NERVE ENDINGS OR GANGLION CELLS... ...ESSENTIALLY SIMILAR ACTION /TO THAT AT CELLULAR LEVEL/ APPEARS TO ACCOUNT FOR SECRETION, AT LEAST AS IT OCCURS IN CHROMAFFIN CELLS OF ADRENAL MEDULLA. HERE HISTAMINE...MIMIC PHYSIOLOGICAL SECRETAGOGUE ACH IN DEPOLARIZING THE PLASMALEMMA. MODE OF ACTION OF HISTAMINE...TO PRODUCE SMOOTH MUSCLE RELAXATION, INCL VASODILATION, HAS NOT BEEN DEFINED. CLASSICAL...ANTIHISTAMINES ARE...CAPABLE OF BLOCKING...H1 RECEPTORS ONLY... H2 RECEPTORS...ARE INHIBITED PREFERENTIALLY BY H2-RECEPTORS ANTAGONISTS. Therapeutic Uses EXPTL USE: IN VET MEDICINE PRIMARILY FOR EXPTL PRODN OF ULCERS IN DOGS & HOGS, & OCCASIONALLY TO DETERMINE STATUS OF HYDROCHLORIC ACID SECRETION IN STOMACH OF DOGS BY USE OF \"DIAGNEX BLUE-TEST\" OR \"GASTRO-TEST\"... EXPTL USE: ...ATTEMPTS HAVE BEEN MADE TO DESENSITIZE PT /TO HISTAMINE ALLERGIES/ WITH COURSES OF HISTAMINE INJECTIONS. THERE IS NO EXPTL EVIDENCE THAT SUCH REGIMES INDUCE SIGNIFICANT TOLERANCE...& PROCEDURE HAS NOT MET WITH GENERAL ACCEPTANCE. PRACTICAL APPLICATIONS OF HISTAMINE FALL INTO TWO CATEGORIES: 1ST, ITS USES AS DIAGNOSTIC AGENT, WHICH FOR MOST PART ARE ON A SOUND PHYSIOLOGICAL BASIS; &, 2ND, ITS MORE CONTROVERSIAL USES IN THERAPY, ESP OF DISEASES OF ALLERGY. AS DIAGNOSTIC AGENT IT IS OF VALUE IN TESTING FOR FUNCTIONAL CAPACITY OF GASTRIC GLANDS. IF NO ACID IS SECRETED FOLLOWING INJECTION OF 0.25-0.5 MG (USUALLY AS 1:1000 SOLN), A TRUE GASTRIC ACHYLIA EXISTS. For more Therapeutic Uses (Complete) data for HISTAMINE (10 total), please visit the HSDB record page. Drug Warnings /WHEN HISTAMINE IS EMPLOYED TOPICALLY TO EYE IN CONCN FROM 0.1 TO 10% IT CAUSES/ VASODILATION & EDEMA OF CONJUNCTIVA. Pharmacodynamics Histamine stimulates gastric gland secretion, causing an increased secretion of gastric juice of high acidity. This action is probably due mainly to a direct action on parietal and chief gland cells. - Histamine levels in synovial fluid of patients with rheumatoid arthritis (RA) were significantly higher than those in healthy controls (12.5 ± 3.2 ng/mL vs. 3.1 ± 0.8 ng/mL). The level of Histamine was positively correlated with the disease activity score (DAS28) of RA patients (r = 0.68, P < 0.01), suggesting that Histamine may be involved in the pathogenesis of RA [1] - Histamine inhibits the growth of Plasmodium falciparum by reducing the availability of intracellular iron in parasitized red blood cells, as adding iron ions (Fe³⁺) at a concentration of 100 μM reversed the inhibitory effect of Histamine (inhibition rate decreased from 85.3% to 32.1%) [2] - In the asthma model, the pro-asthmatic effect of Histamine could be blocked by pretreatment with H1 receptor antagonist (loratadine, 5 mg/kg), which reduced airway resistance by 62.3% and eosinophil count in BALF by 58.7%, indicating that the effect of Histamine is mediated by H1 receptors [3] - Histamine induces gastric and duodenal ulcers by stimulating gastric acid secretion. Pretreatment with cimetidine (a H2 receptor antagonist) at a dose of 20 mg/kg in rats reduced the ulcer area induced by Histamine by 78.5%, confirming the role of H2 receptors in Histamine-induced ulcers [5] |
| 分子式 |
C5H9N3
|
|---|---|
| 分子量 |
111.1451
|
| 精确质量 |
111.079
|
| 元素分析 |
C, 73.52; H, 7.14; N, 9.03; O, 10.31
|
| CAS号 |
51-45-6
|
| 相关CAS号 |
51-45-6; 56-92-8 (HCl); 51-74-1 (phosphate)
|
| PubChem CID |
774
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
331.0±17.0 °C at 760 mmHg
|
| 熔点 |
83-84ºC
|
| 闪点 |
180.3±8.1 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.567
|
| LogP |
-0.92
|
| tPSA |
54.7
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
8
|
| 分子复杂度/Complexity |
64.7
|
| 定义原子立体中心数目 |
0
|
| SMILES |
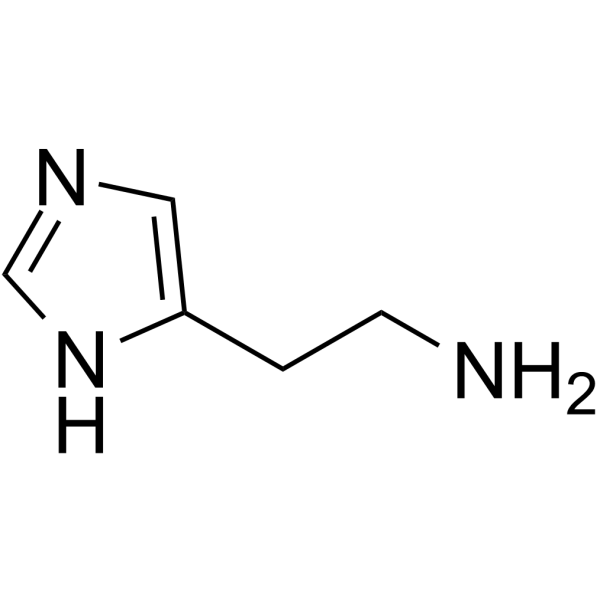
N1([H])C([H])=NC([H])=C1C([H])([H])C([H])([H])N([H])[H]
|
| InChi Key |
NTYJJOPFIAHURM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8)
|
| 化学名 |
2-(1H-imidazol-5-yl)ethanamine
|
| 别名 |
LS82-556; LS 82-556; 2-(1H-imidazol-5-yl)ethanamine; 1H-Imidazole-4-ethanamine; Ergamine; Ergotidine; 2-(4-Imidazolyl)ethylamine; 5-Imidazoleethylamine; LS-82-556
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~22 mg/mL (~197.9 mM)
Water: ~22 mg/mL Ethanol: ~22 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.9969 mL | 44.9843 mL | 89.9685 mL | |
| 5 mM | 1.7994 mL | 8.9969 mL | 17.9937 mL | |
| 10 mM | 0.8997 mL | 4.4984 mL | 8.9969 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00362999 | Active Recruiting |
N/A | Allergic Rhinitis | Children's Mercy Hospital Kansas City |
August 2006 | N/A |
| NCT05131555 | Active Recruiting |
Drug: Placebo: Placebo + exercise training. Drug: H1 blockade: H1 receptor antagonist + exercise training |
Exercise Histamine |
University Ghent | August 16, 2021 | Not Applicable |
| NCT06152497 | Recruiting | Drug: Placebo Behavioral: Resistance training |
Histamine | University Ghent | September 1, 2023 | Not Applicable |
| NCT06154824 | Recruiting | Other: Histamine Other: Cowhage |
Histamine Cowhage |
Aalborg University | December 15, 2023 | Not Applicable |
| NCT06081998 | Recruiting | Other: Histamine Other: Cowhage |
Histamine Cowhage Sleep Deprivation |
Aalborg University | November 1, 2023 | Not Applicable |
|
|
|