| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
(-)-Huperzine A(1 μM;2 小时)可减轻 Aβ23-35 (20 μM) 引起的神经元损伤 [2]。 (-)-Huperzine A (100 μM) 可逆地抑制从大鼠海马中急剧分离的 CA1 锥体神经元的全细胞电压钳记录中 NMDA 诱导的电流 (IC50=126 μM) [3]。
|
|---|---|
| 体内研究 (In Vivo) |
在因静脉注射 β-淀粉样蛋白-(1-40), (-)-石杉碱 A(0.1-0.2 mg/kg;腹腔注射;每天;持续 12 天)引起的变性大鼠中,可减轻神经元损伤和认知功能障碍。 5]。
|
| 动物实验 |
Animal/Disease Models: Male SD (Sprague-Dawley) rats (220-280 g)[5]
Doses: 0.1 mg/kg, 0.2 mg/kg Route of Administration: intraperitoneal (ip)injection, daily, for 12 days Experimental Results: Partly reversed the down-regulation of anti-apoptotic Bcl-2 and the up-regulation of pro-apoptotic Bax and P53 proteins and decreased the apoptosis that normally followed b-amyloid injection; alleviated the cognitive dysfunction induced by b-amyloid protein-(1-40). |
| 参考文献 |
|
| 其他信息 |
Huperzine A is a sesquiterpene alkaloid isolated from a club moss Huperzia serrata that has been shown to exhibit neuroprotective activity. It is also an effective inhibitor of acetylcholinesterase and has attracted interest as a therapeutic candidate for Alzheimer's disease. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a neuroprotective agent, a plant metabolite and a nootropic agent. It is a sesquiterpene alkaloid, a pyridone, a primary amino compound and an organic heterotricyclic compound. It is a conjugate base of a huperzine A(1+).
Huperzine A, is a naturally occurring sesquiterpene alkaloid found in the extracts of the firmoss Huperzia serrata. The botanical has been used in China for centuries for the treatment of swelling, fever and blood disorders. Recently in clinical trials in China, it has demonstrated neuroprotective effects. It is currently being investigated as a possible treatment for diseases characterized by neurodegeneration – particularly Alzheimer’s disease. Huperzine A has been reported in Aspergillus versicolor, Phlegmariurus phlegmaria, and other organisms with data available. Drug Indication Investigated for use/treatment in alzheimer's disease. Mechanism of Action Huperzine A has been found to be an inhibitor of the enzyme acetylcholinesterase. This is the same mechanism of action of pharmaceutical drugs such as [galantamine] and [donepezil] used to treat Alzheimer's disease. Pharmacodynamics Huperzine A is an alkaloid derived from Huperzia serrata (which is available as an herbal product in the US). It is under investigation as an acetylcholinesterase inhibitor. Clinical trials in China have shown that huperzine A is comparably effective to the drugs currently on the market, and may even be somewhat safer in terms of side effects. |
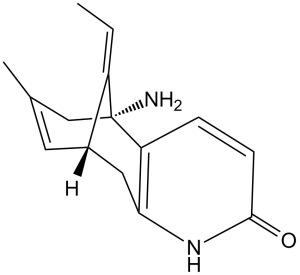
| 分子式 |
C15H18N2O
|
|
|---|---|---|
| 分子量 |
242.32
|
|
| 精确质量 |
242.141
|
|
| CAS号 |
102518-79-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
854026
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.6±0.1 g/cm3
|
|
| 沸点 |
479.5±25.0 °C at 760 mmHg
|
|
| 熔点 |
211-216oC
|
|
| 闪点 |
243.8±23.2 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.741
|
|
| LogP |
-0.22
|
|
| tPSA |
58.88
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
551
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
C/C=C/1\[C@@H]2CC3=C([C@]1(CC(=C2)C)N)C=CC(=O)N3
|
|
| InChi Key |
ZRJBHWIHUMBLCN-YQEJDHNASA-N
|
|
| InChi Code |
InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1
|
|
| 化学名 |
(1R,9R,13E)-1-amino-13-ethylidene-11-methyl-6-azatricyclo[7.3.1.02,7]trideca-2(7),3,10-trien-5-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.32 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.32 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.32 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W:30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1268 mL | 20.6339 mL | 41.2677 mL | |
| 5 mM | 0.8254 mL | 4.1268 mL | 8.2535 mL | |
| 10 mM | 0.4127 mL | 2.0634 mL | 4.1268 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01676311 | Terminated Has Results | Drug: Huperzine A Drug: Placebo |
Traumatic Brain Injury | Spaulding Rehabilitation Hospital | December 2013 | Phase 2 |
| NCT01194336 | Completed | Drug: Huperzine A Drug: Donepezil Drug: Galantamine Other: Placebo |
Biomarkers, Pharmacological | U.S. Army Medical Research and Development Command |
February 2012 | |
| NCT05518578 | Recruiting | Drug: SPN-817 | Epilepsy Seizures, Epileptic |
Supernus Pharmaceuticals, Inc. | February 7, 2023 | Phase 2 |
| NCT01136551 | Unknown † | Drug: Huperzine A | Healthy Bioavailability |
Hadassah Medical Organization | September 2010 | Not Applicable |
| NCT01282619 | Unknown † | Drug: Huperzine A Drug: huperzine A Drug: Placebo |
Alzheimer's Disease | Shandong Luye Pharmaceutical Co., Ltd. | May 2010 | Phase 2 Phase 3 |
|
|---|
|
 |