| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |

| 体外研究 (In Vitro) |
布洛芬 (24 h) 抑制 COX-1 和 COX-2 活性,IC50 值为 13 μM 和 370 μM[1]。在 AGS 细胞(胃腺癌细胞系)中,布洛芬(500 μM,48 小时)会导致细胞凋亡并抑制血管生成和细胞增殖[2]。在 AGS 细胞中,布洛芬(500 μM,48 小时)会增加野生型 P53 和 Bax 基因的 RNA 水平,但下调 Akt、VEGF-A、PCNA、Bcl2、OCT3/4 和 CD44 基因的转录[2]。在原代 CF 鼻上皮细胞和囊性纤维化 (CF) 细胞模型中,布洛芬(500 μM,24 小时)可导致微管延伸至细胞外周,并恢复微管依赖性细胞内胆固醇转运[3]。通过光敏化过程,布洛芬(500 μM,24 小时)会增加 MCF-7 和 MDA-MB-231 细胞中紫外线诱导的细胞死亡[4]。
|
|---|---|
| 体内研究 (In Vivo) |
在产后乳腺癌模型中,布洛芬(300 mg/kg;口服;每天,持续 14 天)可减少总体肿瘤生长并改善抗肿瘤免疫功能,而不会引起有害的自身免疫反应[5]。 ?在慢性奥沙利铂模型的大鼠中,布洛芬(60 mg/kg;ih;每隔一天,持续 15 天)是否会降低导致周围神经病变的神经病变风险[6]。布洛芬(20 mg/kg;口服;每12小时一次,共5剂)可降低平均肌纤维横截面积,但不会改变冈上肌腱对运动的适应调节[7]。 ?在持续性肺部感染的大鼠模型中,布洛芬(35 mg/kg;口服;每日两次)可减少对铜绿假单胞菌的炎症反应[8]。
|
| 细胞实验 |
细胞活力测定[2]
细胞类型: AGS 细胞 测试浓度: 100-1000 μM 孵育时间: > 24 小时、48 小时 实验结果: 抑制 AGS 细胞活力,IC50 值为 630 μM(台盼蓝染色,24 小时)、456 μM(中性红测定,24 小时)、549 μM(台盼蓝染色,48 小时)和 408 μM(中性红测定,48 小时)。 |
| 动物实验 |
Animal/Disease Models: Syngeneic (D2A1) orthotopic Balb/c mouse model of PPBC (postpartum)[5]
Doses: 300 mg/kg, daily for 14 days Route of Administration: Fed in animal feedings (added to pulverized standard chow and mixed dry , then mixed with water, made into chow pellets and dried thoroughly) Experimental Results: Suppresed tumor growth, decreased presence of immature monocytes and increased numbers of T cells. Enhanced Th1 associated cytokines as well as promoted tumor border accumulation of T cells. Animal/Disease Models: Oxaliplatin‑induced peripheral neuropathy[6] Doses: 60 mg/kg, every second day for 15 days Route of Administration: subcutaneous (sc) injection Experimental Results: Lowered sensory nerve conduction velocity (SNCV). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
It is very well absorbed orally and the peak serum concentration can be attained in 1 to 2 hours after extravascular administration. When ibuprofen is administered immediately after a meal there is a slight reduction in the absorption rate but there is no change in the extent of the absorption. When orally administered, the absorption of ibuprofen in adults is very rapidly done in the upper GI tract. The average Cmax, Tmax and AUC ranges around 20 mcg/ml, 2 h and 70 mcg.h/ml. These parameters can vary depending on the enantiomer form, route, and dose of administration. Ibuprofen is rapidly metabolized and eliminated in the urine thus, this via accounts for more than 90% of the administered dose. It is completely eliminated in 24 hours after the last dose and almost all the administered dose goes through metabolism, representing about 99% of the eliminated dose. The biliary excretion of unchanged drug and active phase II metabolites represents 1% of the administered dose. In summary, ibuprofen is excreted as metabolites or their conjugates. The elimination of ibuprofen is not impaired by old age or the presence of renal impairment. The apparent volume of distribution of ibuprofen is of 0.1 L/kg. The clearance rate ranges between 3-13 L/h depending on the route of administration, enantiomer type and dosage. Ibuprofen is rapidly absorbed after oral admin, & peak concns in plasma are observed after 15-30 min. The half-life in plasma is about 2 hr. Ibuprofen is extensively (99%) bound to plasma proteins, but the drug occupies only a fraction of the total drug-binding sites at usual concns. Ibuprofen passes slowly into the synovial spaces & may remain there in higher concn as the concns in plasma decline. In experimental animals, ibuprofen & its metabolites pass easily across the placenta. The excretion of ibuprofen is rapid & complete. More than 90% of an ingested dose is excreted in the urine as metabolites or their conjugates. ENTERO-HEPATIC CIRCULATION OF (14)C-IBUPROFEN & ITS METABOLITES MAY HAVE OCCURRED IN DOGS RECEIVING REPEATED ORAL DOSES ... SINCE LEVELS IN BILE ... WERE 40-FOLD THOSE IN PLASMA. AFTER ORAL DOSES OF 400 MG IBUPROFEN, SERIAL BLOOD SAMPLES WERE TAKEN (5 MALE VOLUNTEERS, 4 ARTHRITIC PATIENT). EVIDENCE SHOWED 2 COMPARTMENT MODEL: NO EVIDENCE SHOWN OF DRUG ACCUM IN PERIPHERAL COMPARTMENT. The enantiomeric composition of ibuprofen in plasma was investigated following oral administration of 200 mg of the racemic drug in a conventional tablet or 300 mg in a novel controlled release pellet formulation to 4 healthy volunteers, aged 24 to 37 yr, in a randomized, crossover study. The plasma concentration time profiles suggest that drug release from the controlled release preparation was suitably modified and that the fluctuation between the peaks and troughs observed following a conventional tablet formulation were reduced. The plasma concentrations of (+)-ibuprofen (S-ibuprofen) were greater than those of (-)-ibuprofen (R-ibuprofen) following either formulation, and the enantiomeric plasma ratio (S/R) was reduced, both in magnitude and variability, following the controlled release preparation. The proportion of the total area under the plasma concentration time curves, due to (S)-ibuprofen, were slightly reduced following the controlled release formulation compared to the tablet formulation. The importance of a consideration of stereochemistry in bioequivalence studies of chiral drugs is discussed. For more Absorption, Distribution and Excretion (Complete) data for IBUPROFEN (11 total), please visit the HSDB record page. Metabolism / Metabolites Ibuprofen is rapidly metabolized and biotransformed in the liver to the formation of major metabolites which are the hydroxylated and carboxylated derivatives. As soon as it is absorbed, the R-enantiomer undergoes extensive enantiomeric conversion (53-65%) to the more active S-enantiomer _in vivo_ by the activity of alpha-methylacyl-CoA racemase. Ibuprofen metabolism can be divided in phase I which is represented by the hydroxylation of the isobutyl chains for the formation of 2 or 3-hydroxy derivatives followed by oxidation to 2-carboxy-ibuprofen and p-carboxy-2-propionate. These oxidative reactions are performed by the activity of the cytochrome P450 isoforms CYP 2C9, CYP 2C19 and CYP 2C8. Therefore, these enzymes participate in the oxidation of the alkyl side chain to hydroxyl and carboxyl derivatives. From this enzymes, the major catalyst in the formation of oxidative metabolites is the isoform CYP 2C9. The metabolic phase I is followed by a phase II in which the oxidative metabolites may be conjugated to glucuronide prior to excretion. This activity forms phenolic and acyl glucuronides. TWO MAJOR METABOLIC PATHWAYS IN MAN & IN ANIMALS PROCEED BY OXIDATIVE ATTACK OF ISOBUTYL SIDE CHAIN; THEY ARE HYDROXYLATION OF THE TERTIARY CARBON TO YIELD A STABLE TERTIARY ALCOHOL, & OXIDATION OF 1 OF THE 2 GEMINAL METHYL GROUPS TO YIELD THE ACID. IBUPROFEN GIVES 2-(4-(2-CARBOXYPROPYL)PHENYL)PROPIONIC ACID & 2-(4-(2-HYDROXY-2-METHYLPROPYL)PHENYL)PROPIONIC ACID IN MAN. /FROM TABLE/ The pharmacokinetics of oral ibuprofen following a dose of 0.8 g given 3 times a day for 14 days were studied in 7 functionally anephric patients (aged 34-66 yr) undergoing hemodialysis. No accumulation of ibuprofen plasma concns & an absence of intact ibuprofen in dialysate indicated clearance through metabolic pathways. The metabolites did accumulate significantly with mean plasma levels of 249 mcg/ml for the carboxy derivatives & 57 mcg/ml for the hydroxy derivatives of ibuprofen. However, both were detected in the dialysate. Dialysis clearance calculated by arterial & venous difference was found to agree with actual recovery in dialysate for both metabolites. Side effects were not observed in any subject. R-enanatiomer undergoes extensive enantiomeric conversion (53-65%) to the more active S-enantiomer in vivo. Metablized by oxidation to 2 inactive metabolites: (+)-2[4'-(2-hydroxy-2-methylpropyl)phenyl]propionic acid and (+)-2-[4'-(2-carboxypropyl)phenyl]propionic acid. Very small amounts of 1-hydroxyibuprofen and 3-hydroxyibuprofen have been recovered from urine. Cytochrome P450 2C9 is the major catalyst in the formation of oxidative metabolites. Oxidative metabolites may be conjugated to glucuronide prior to excretion. Route of Elimination: Ibuprofen is rapidly metabolized and eliminated in the urine. Half Life: 2-4 hours Biological Half-Life The serum half-life of ibuprofen is 1.2-2 hours. In patients with a compromised liver function, the half-life can be prolonged to 3.1-3.4 hours. ... After oral admin ... the half-life in plasma is about 2 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The exact mechanism of action of ibuprofen is unknown. Ibuprofen is a non-selective inhibitor of cyclooxygenase, an enzyme invovled in prostaglandin synthesis via the arachidonic acid pathway. Its pharmacological effects are believed to be due to inhibition cylooxygenase-2 (COX-2) which decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever and swelling. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation. Inhibition of COX-1 is thought to cause some of the side effects of ibuprofen including GI ulceration. Ibuprofen is administered as a racemic mixture. The R-enantiomer undergoes extensive interconversion to the S-enantiomer in vivo. The S-enantiomer is believed to be the more pharmacologically active enantiomer. Toxicity Data LD50: 1255mg/kg (Oral, Mouse) (A308) Interactions IN RABBITS AND IN HEALTHY HUMANS, IBUPROFEN ADMINISTERED BEFORE TOLBUTAMIDE ANTAGONIZED TOLBUTAMIDE HYPOGLYCEMIA. WHEN SULFAMETHIZOLE WAS COADMINISTERED TO DOGS WITH IBUPROFEN, BETA-ELIMINATION HALF-LIFE FOR SULFAMETHIZOLE WAS INCREASED APPROXIMATELY 10 TIMES COMPARED TO THE CONTROL VALUE. RESULTS SUGGEST THAT THE INCREASED TERMINAL HALF-LIFE OF SULFAMETHIZOLE CAUSED BY IBUPROFEN IS MAINLY A RESULT OF COMPETITIVE INTERACTIONS BETWEEN THEM AT THE RENAL SECRETORY LEVEL. In several short-term, controlled studies, ibuprofen did not have a substantial effect on the prothrombin time of patients receiving oral anticoagulants; however, because ibuprofen may cause GI bleeding, inhibit platelet aggregation, and prolong bleeding time and because bleeding has occurred when ibuprofen and coumarin derivative anticoagulants were administered concomitantly, the drug should be used with caution and the patient carefully observed if the drug is used concomitantly with any anticoagulant /such as/ warfarin or /the thrombolytic agent streptokinase. To study a potential interaction between digoxin and two non-steroid anti-inflammatory drugs, indomethacin (50 mg three times daily) and ibuprofen (600 mg three times daily) were given for 10 days to 10 and 8 patients, respectively, on chronic digoxin treatment. Serum digoxin measured by fluorescence polarisation immunoassay increased significantly (p < 0.05) during treatment with indomethacin from pre-treatment values of 0.73 + or - 0.34 nmol/l (mean + or - standard deviation) to a mean value of 1.02 + or - 0.43 nmol/l, while administration of ibuprofen did not change the steady state serum concentration of digoxin. The result demonstrates that some non-steroidal anti-inflammatory drugs such as indomethacin increase serum digoxin to levels high in the therapeutic range. This should be taken into consideration when co-administering other drugs known to increase the serum concentration of digoxin such as several antiarrhythmics. For more Interactions (Complete) data for IBUPROFEN (17 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 636 mg/kg LD50 Rat ip 626 mg/kg LD50 Rat sc 740 mg/kg LD50 Rat rectal 530 mg/kg For more Non-Human Toxicity Values (Complete) data for IBUPROFEN (8 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Noreen Y, et al. Development of a radiochemical cyclooxygenase-1 and -2 in vitro assay for identification of natural products as inhibitors of prostaglandin biosynthesis. J Nat Prod. 1998 Jan;61(1):2-7.
[2]. Hassan Akrami, et al. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015 May;36(5):3237-43. [3]. Sharon M Rymut, et al. Ibuprofen regulation of microtubule dynamics in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2016 Aug 1;311(2):L317-27. [4]. Emmanuelle Bignon, et al. Ibuprofen and ketoprofen potentiate UVA-induced cell death by a photosensitization process. Sci Rep. 2017 Aug 21;7(1):8885. [5]. Nathan D Pennock, et al. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J Immunother Cancer. 2018 Oct 1;6(1):98. [6]. Thomas Krøigård, et al. Protective effect of ibuprofen in a rat model of chronic oxaliplatin-induced peripheral neuropathy. Exp Brain Res. 2019 Oct;237(10):2645-2651. [7]. Sarah Ilkhanipour Rooney, et al. Ibuprofen Differentially Affects Supraspinatus Muscle and Tendon Adaptations to Exercise in a Rat Model. Am J Sports Med. 2016 Sep;44(9):2237-45. [8]. M W Konstan, et al. Ibuprofen attenuates the inflammatory response to Pseudomonas aeruginosa in a rat model of chronic pulmonary infection. Implications for antiinflammatory therapy in cystic fibrosis. Am Rev Respir Dis. 1990 Jan;141(1):186-92. |
| 其他信息 |
Therapeutic Uses
Analgesics, Non-Narcotic; Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors Ibuprofen ... /is/ indicated for reduction of fever. /Included in US product labeling/ Ibuprofen ... /is/ used for relief of the pain and inflammation of acute gouty arthritis and acute calcium pyrophosphate deposition disease (pseudogout; chondrocalcinosis articularis; synovitis, crystal-induced). Only immediate-release dosage forms are recommended for relief of acute attacks because of their more rapid onset of action relative to delayed-release or extended-release dosage forms. /NOT included in US product labeling/ Ibuprofen ... /is/ indicated for relief of mild to moderate pain, especially when anti-inflammatory actions may also be desired, e.g., following dental, obstetric, or orthopedic surgery, and for relief of musculoskeletal pain due to soft tissue athletic injuries (strains or sprains). Only immediate-release dosage forms are recommended for relief of acute pain because of their more rapid onset of actin relative to delayed-release or extended-release dosage forms. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for IBUPROFEN (28 total), please visit the HSDB record page. Drug Warnings Ibuprofen should be used with caution in patients with peptic ulcer disease, GI perforation or bleeding, bleeding abnormalities (especially in patients who may be adversely affected by prolongation of bleeding time), impaired renal function, hypertension, or compromised cardiac function. CAUTION IN THE USE OF IBUPROFEN IN SYSTEMIC LUPUS ERYTHEMATOSUS IS ADVISED, PARTICULARLY IF THERE IS HISTORY OF SALICYLATE INTOLERANCE. Ibuprofen is not recommended for use by pregnant women, or by those who are breast-feeding their infants. IBUPROFEN ELEVATES BILIRUBIN, ALKALINE PHOSPHATASE, ASPARTATE AMINOTRANSFERASE (SGOT), AND ALANINE AMINOTRANSFERASE (SGPT) ABOVE THE NORMAL RANGE, AND CAUSES ISOLATED CASES OF JAUNDICE. /FROM TABLE/ For more Drug Warnings (Complete) data for IBUPROFEN (14 total), please visit the HSDB record page. Pharmacodynamics Ibuprofen has multiple actions in different inflammatory pathways involved in acute and chronic inflammation. The main effects reported in ibuprofen are related to the control of pain, fever and acute inflammation by the inhibition of the synthesis of prostanoids by COX-1 and COX-2. Pain relief is attributed to peripheral affected regions and central nervous system effects in the pain transmission mediated by the dorsal horn and higher spinothalamic tract. Some reports have tried to link the pain regulation with a possible enhancement on the synthesis of endogenous cannabinoids and action on the NMDA receptors. The effect on pain has been shown to be related to the cortically evoked potentials. The antipyretic effect is reported to be linked to the effect on the prostanoid synthesis due to the fact that the prostanoids are the main signaling mediator of pyresis in the hypothalamic-preoptic region. The use of ibuprofen in dental procedures is attributed to the local inhibition of prostanoid production as well as to anti-oedemic activity and an increase of plasma beta-endorphins. Some reports have suggested a rapid local reduction of the expression of COX-2 in dental pulp derived by the administration of ibuprofen. The administration of ibuprofen in patients with rheumatic diseases has shown to control joint symptoms. Ibuprofen is largely used in OTC products such as an agent for the management of dysmenorrhea which has been proven to reduce the amount of menstrual prostanoids and to produce a reduction in the uterine hypercontractility. As well, it has been reported to reduce significantly the fever and the pain caused by migraines. This effect is thought to be related to the effect on platelet activation and thromboxane A2 production which produces local vascular effects in the affected regions. This effect is viable as ibuprofen can enter in the central nervous system. In the investigational uses of ibuprofen, it has been reported to reduce neurodegeneration when given in low doses over a long time. On the other hand, its use in Parkinson disease is related to the importance of inflammation and oxidative stress in the pathology of this condition. The use of ibuprofen for breast cancer is related to a study that shows a decrease of 50% in the rate of breast cancer. |
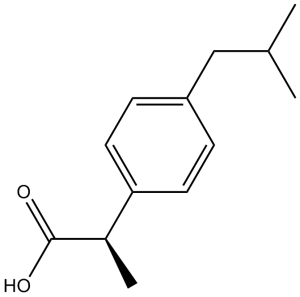
| 分子式 |
C13H18O2
|
|---|---|
| 分子量 |
206.28
|
| 精确质量 |
206.13
|
| CAS号 |
15687-27-1
|
| 相关CAS号 |
(S)-(+)-Ibuprofen;51146-56-6;(S)-(+)-Ibuprofen-d3;1329643-44-8;(R)-(-)-Ibuprofen;51146-57-7;Ibuprofen sodium;31121-93-4;Ibuprofen-d3;121662-14-4;Ibuprofen-d4;Ibuprofen-13C6;1216459-54-9;Ibuprofen L-lysine;57469-77-9;Ibuprofen-13C,d3;1261394-40-4
|
| PubChem CID |
3672
|
| 外观&性状 |
Colorless, crystalline stable solid
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
319.6±11.0 °C at 760 mmHg
|
| 熔点 |
77-78 °C(lit.)
|
| 闪点 |
216.7±14.4 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.519
|
| LogP |
3.72
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
203
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C(C([H])(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])=O
|
| InChi Key |
HEFNNWSXXWATRW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
|
| 化学名 |
2-[4-(2-methylpropyl)phenyl]propanoic acid
|
| 别名 |
Ibuprofen, Advil, Motrin, Nurofen, Brufen
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 20 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8478 mL | 24.2389 mL | 48.4778 mL | |
| 5 mM | 0.9696 mL | 4.8478 mL | 9.6956 mL | |
| 10 mM | 0.4848 mL | 2.4239 mL | 4.8478 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06247462 | Completed | Drug: Ibuprofen 600 mg Drug: Placebo Corn Starch |
Acute Kidney Injury | University of New Mexico | June 1, 2023 | Phase 1 |
| NCT05401916 | Completed | Drug: Paracetamol Drug: Ibuprofen |
Postoperative Pain Management | Bezmialem Vakif University | June 10, 2022 | Not Applicable |
| NCT05971186 | Completed | Drug: Ibuprofen 400 mg Other: Young Coconut Water |
Menstrual Pain Ibuprofen |
Universitas Padjadjaran | June 30, 2022 | Phase 2 |
| NCT02538237 | Completed | Drug: placebo Drug: Ibuprofen 2% mouthwash |
Periodontitis | Islamic Azad University, Tehran | November 2013 | Phase 2 Phase 3 |