| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
EGFR (IC50 = 5 nM); EGFRL858R; EGFRL858R/T790M; EGFRT790M; EGFRL861Q
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:埃克替尼以剂量依赖性方式抑制EGFR活性,IC50值为5 nM,在62.5 nM时完全抑制。埃克替尼选择性地单独抑制EGFR成员,包括野生型和突变型,抑制效率为61-99%。埃克替尼以剂量依赖性方式阻断人表皮样癌 A431 细胞中 EGFR 介导的细胞内酪氨酸磷酸化。同时,在我们对A431、BGC-823、A549、H460、HCT8、KB和Bel-7402细胞系进行的增殖测定中,我们发现细胞系对埃克替尼的相对敏感性为A431 > BGC-823 > A549 > H460 >知识库 > HCT8 和 Bel-7402。埃克替尼表现出广泛的抗肿瘤活性,尤其对表达较高水平 EGFR 的肿瘤特别有效。激酶测定:在体外激酶测定中,2.4 ng/μL EGFR 蛋白与 32 ng/μL Crk 在含有 1 μM 冷 ATP 和 1 μCi 32P-γ-ATP 的 25 μL 激酶反应缓冲液中混合。将混合物与 0、0.5、2.5、12.5 或 62.5 nM 埃克替尼在冰上孵育 10 分钟,然后在 30 °C 下孵育 20 分钟。在 100 °C 下用 SDS 样品缓冲液淬灭 4 分钟后,通过在 10% SDS-PAGE 凝胶中电泳来解析蛋白质混合物。然后将干燥的凝胶暴露于 PhosphorImager 以检测放射性。定量由 ImageQuant 软件执行。在该方法中,放射性信号与激酶活性成反比。细胞测定:将细胞(103 个/孔,A431、BGC-823、A549、H460、HCT8、KB 和 Bel-7402 细胞)接种到 96 孔板的含有 10% FBS 的 RPMI-1640 培养基中,并在 5 % CO2 培养箱,37°C。 24小时后,用0、0.78、1.56、3.125、6.25、12.5或25μM埃克替尼处理细胞96小时。通过从第 4 天的平均吸光度值减去第 0 天的平均吸光度值来计算细胞增殖。
|
| 体内研究 (In Vivo) |
体内:体内研究表明,埃克替尼在携带各种人类肿瘤衍生异种移植物的裸鼠体内表现出强烈的剂量依赖性抗肿瘤作用。该药物在高达120mg/kg/天的剂量下对小鼠具有良好的耐受性,在治疗过程中没有死亡或显著的体重减轻。最近完成了一项使用吉非替尼作为晚期癌症(NSCLC)患者主动对照的头对头随机双盲III期试验(试验注册ID:NCT01040780)。
埃克替尼在不同类型的异种移植物中显示出抗肿瘤作用。埃克替尼在 120 mg/kg 的剂量下,对 A431、A549 和 H460 异种移植物的肿瘤生长抑制率分别为 51.5%、31.0% 和 67.4%。 |
| 酶活实验 |
将 2.4 ng/μL EGFR 蛋白和 32 ng/μL Crk 混合在 25 μL 激酶反应缓冲液中,其中含有 1 μM 冷 ATP 和 1 μCi32P-γ-ATP,用于体外激酶测定。使用 10 分钟的冰基孵育时间在 0、0.5、2.5、12.5 或 62.5 nM 埃克替尼下孵育混合物。然后添加 20 分钟的固化时间。在 100°C 下用 SDS 样品缓冲液淬灭 4 分钟后,使用 10% SDS-PAGE 凝胶进行电泳以解析蛋白质混合物。为了检测放射性,随后将干燥的凝胶暴露。软件处理量化[1]。
|
| 细胞实验 |
在含有 10% FBS 的 RPMI-1640 培养基中,将每孔 1000 个细胞接种到 96 孔板中。然后细胞在 37°C、5% CO2 的培养箱中生长。一天后,将埃克替尼以 0、0.78、1.56、3.125、6.25、12.5 或 25 μM 添加到细胞中,持续一整天。细胞增殖的计算包括从第4天的平均吸光度值减去第0天的平均吸光度值[1]。
|
| 动物实验 |
Mice: In mice with A431, A549, H460, and HCT8 tumor xenografts, the effects of three doses of icotinib (30, 60, and 120 mg/kg/dose p.o. qd) on antitumor activity and survival are assessed. In these studies, a positive control group is given 30 mg/kg/dose intraperitoneally once a week[1].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability = 52% >90% via faeces, 9% via urine the volume of distribution was calculated as Vz/F = 115.00 ± 63.26 l the clearance was calculated as CL/F = 13.30 ± 4.78 l/h Metabolism / Metabolites Hepatic (mainly CYP3A4, less CYP1A2) Biological Half-Life 5.5 hrs |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Icotinib binds to Sudlow's site I in subdomain IIA of Human Serum Albumin (HSA) molecule, resulting in the formation of icotinib-HSA complexes. |
| 参考文献 | |
| 其他信息 |
Icotinib is a potent and specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) Icotinib was approved in China by the SFDA in June, 2011 and in January 2014, Beta Pharma, Inc. was given a “May Proceed” from the US FDA to conduct a Phase I study for the evaluation of icotinib as a treatment of EGFR+ Non-Small Cell Lung Cancer (NSCLC).
Icotinib is an orally available quinazoline-based inhibitor of epidermal growth factor receptor (EGFR), with potential antineoplastic activity. Icotinib selectively inhibits the wild-type and several mutated forms of EGFR tyrosine kinase. This may lead to an inhibition of EGFR-mediated signal transduction and may inhibit cancer cell proliferation. EGFR, a receptor tyrosine kinase, has been upregulated in a variety of cancer cell types. Drug Indication Icotinib hydrochloride is a novel epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitor, exhibits encouraging efficacy and tolerability in patients with advanced non-small-cell lung cancer (NSCLC) who failed previous chemotherapy. Mechanism of Action Icotinib is a highly selective, first generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) which binds reversibly to the ATP binding site of the EGFR protein, preventing completion of the signal transduction cascade. EGFR is an oncogenic receptor and patients with activating somatic mutations, such as an exon 19 deletion or exon 21 L858R mutation, within the tyrosine kinase domain display unchecked cell proliferation. Icotinib hydrochloride is a member of quinazolines. Icotinib Hydrochloride is the hydrochloride salt form of icotinib, an orally available quinazoline-based inhibitor of epidermal growth factor receptor (EGFR), with potential antineoplastic activity. Icotinib selectively inhibits the wild-type and several mutated forms of EGFR tyrosine kinase. This may lead to an inhibition of EGFR-mediated signal transduction and may inhibit cancer cell proliferation. EGFR, a receptor tyrosine kinase, has been upregulated in a variety of cancer cell types. Icotinib, one of the leading compounds selected from our compound library, was found to be a potent and specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with an IC(50) of 5 nM. When profiled with 88 kinases, Icotinib only showed meaningful inhibitory activity to EGFR and its mutants. Icotinib blocked EGFR-mediated intracellular tyrosine phosphorylation (IC(50)=45 nM) in the human epidermoid carcinoma A431 cell line and inhibits tumor cell proliferation. In vivo studies demonstrated that Icotinib exhibited potent dose-dependent antitumor effects in nude mice carrying a variety of human tumor-derived xenografts. The drug was well tolerated at doses up to 120 mg/kg/day in mice without mortality or significant body weight loss during the treatment. A head to head randomized, double blind phase III trial using Gefitinib as an active control for patients with advanced non-small cell lung cancer (NSCLC) was finished recently (Trial registration ID: NCT01040780). The data shows that Icotinib was non-inferior to Gefitinib in terms of median progression free survival (PFS) and safety superior favor to Icotinib compared to Gefitinib.[1] |
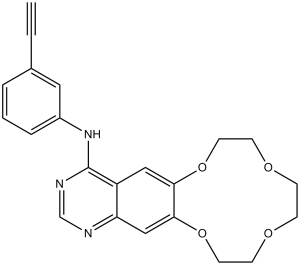
| 分子式 |
C22H21N3O4
|
|---|---|
| 分子量 |
391.42
|
| 精确质量 |
391.153
|
| 元素分析 |
C, 67.51; H, 5.41; N, 10.74; O, 16.35
|
| CAS号 |
610798-31-7
|
| 相关CAS号 |
Icotinib Hydrochloride;1204313-51-8;Icotinib-d4;1567366-82-8
|
| PubChem CID |
22024915
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
581.3±50.0 °C at 760 mmHg
|
| 闪点 |
305.3±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.649
|
| LogP |
1.88
|
| tPSA |
74.73
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
553
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O1C([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC([H])([H])C([H])([H])OC2C([H])=C3C(C(=NC([H])=N3)N([H])C3=C([H])C([H])=C([H])C(C#C[H])=C3[H])=C([H])C1=2
|
| InChi Key |
QQLKULDARVNMAL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H21N3O4/c1-2-16-4-3-5-17(12-16)25-22-18-13-20-21(14-19(18)23-15-24-22)29-11-9-27-7-6-26-8-10-28-20/h1,3-5,12-15H,6-11H2,(H,23,24,25)
|
| 化学名 |
N-(3-ethynylphenyl)-2,5,8,11-tetraoxa-15,17-diazatricyclo[10.8.0.014,19]icosa-1(12),13,15,17,19-pentaen-18-amine
|
| 别名 |
BPI-2009; BPI2009; BPI 2009; BPI2009H; BPI-2009H; 610798-31-7; Conmana; BPI-2009; N-(3-ethynylphenyl)-7,8,10,11,13,14-hexahydro-[1,4,7,10]tetraoxacyclododecino[2,3-g]quinazolin-4-amine; UNII-9G6U5L461Q; 9G6U5L461Q; ICOTINIB [WHO-DD]; BPI 2009H; Icotinib; Trade name: Conmana
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 0.5% CMC: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5548 mL | 12.7740 mL | 25.5480 mL | |
| 5 mM | 0.5110 mL | 2.5548 mL | 5.1096 mL | |
| 10 mM | 0.2555 mL | 1.2774 mL | 2.5548 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04206072 | Active Recruiting |
Drug: Icotinib Hydrochloride Tablets Drug: D-0316 Capsule |
Non-Small Cell Lung Cancer EGFR Gene Mutation |
Betta Pharmaceuticals Co., Ltd. | December 24, 2019 | Phase 2 Phase 3 |
| NCT02448797 | Active Recruiting |
Drug: Icotinib Drug: Chemotherapy |
Non-small Cell Lung Cancer | Betta Pharmaceuticals Co., Ltd | June 8, 2015 | Phase 3 |
| NCT02264210 | Recruiting | Drug: Icotinib | Lung Neoplasms Large Cell Lung Cancer |
Sun Yat-sen University | January 2015 | Phase 2 |
| NCT06041776 | Recruiting | Drug: Befotertinib + Icotinib placebo Drug: Icotinib + Befotertinib placebo |
Adjuvant Therapy EGFR Sensitive Mutation |
Betta Pharmaceuticals Co., Ltd. | March 28, 2023 | Phase 3 |
| NCT03992885 | Recruiting | Drug: Icotinib | Non-squamous Non-small Cell Lung Cancer |
Tianjin Medical University Cancer Institute and Hospital |
July 1, 2019 | Phase 3 |