| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
| 靶点 |
Muscarinic acetylcholine receptors (M1-M5), Ki values for M1 (1.6 nM), M2 (2.5 nM), M3 (1.2 nM), M4 (1.8 nM), M5 (2.1 nM) [3]
- Vagal nerve-mediated muscarinic receptors in guinea-pig airways [1] - Muscarinic receptors involved in pulmonary inflammatory response regulation in rats [4] |
|---|---|
| 体外研究 (In Vitro) |
异丙托溴铵(1 nM、10 nM、100 nM;15 分钟)的毒性作用是由线粒体膜电位破坏引起的[1]。在离体灌注心脏的缺血/再灌注试验中,异丙托溴铵(1 nM-1 μM;4 小时)以剂量反应方式增加梗塞面积 (EC50=22.7 nM) [1]。异丙托溴铵(0.001 nM-0.1 mM;2 小时)可抑制在缺氧条件下生长 4 小时的成年大鼠心肌细胞的生长[1]。
在心肌缺血再灌注体外模型中,异丙托溴铵(浓度未明确)处理可诱导心肌损伤,表现为心肌细胞死亡增加、乳酸脱氢酶(LDH)释放升高及细胞活力下降 [2] - 异丙托溴铵在体外对毒蕈碱型乙酰胆碱受体具有强效竞争性拮抗作用,对M3亚型亲和力最高(Ki=1.2 nM),其次为M1、M4、M5和M2亚型 [3] |
| 体内研究 (In Vivo) |
异丙托溴铵(1.0 μg/kg;静脉注射;单剂量)可放大迷走神经刺激引起的支气管收缩的效果[2]。通过降低中性粒细胞实质炎症浸润炎症,异丙托溴铵(0.04 mg/20 mL 和 0.20 mg/20 mL;30 分钟,速率=30 mL/30 分钟)可保护肺部免受镉诱导的急性中性粒细胞浸润[4]。
在豚鼠体内,静脉注射异丙托溴铵(1 μg/kg、3 μg/kg、10 μg/kg)可呈剂量依赖性增强迷走神经刺激诱导的支气管收缩;10 μg/kg剂量时 potentiation 效应最强,支气管阻力较对照组增加2.3倍 [1] - 在镉诱导的急性肺炎症大鼠模型中,气管内给予异丙托溴铵(0.1 mg/kg)可显著减轻肺炎症反应,包括减少肺组织中中性粒细胞浸润、降低支气管肺泡灌洗液(BALF)中促炎细胞因子(TNF-α、IL-1β、IL-6)水平及减轻肺组织水肿 [4] |
| 酶活实验 |
毒蕈碱型乙酰胆碱受体结合实验:从相应组织中制备富含M1-M5受体的膜组分,将其与系列浓度的异丙托溴铵在放射性标记毒蕈碱激动剂存在下共同孵育。孵育后通过玻璃纤维滤膜真空过滤去除未结合配体,采用闪烁计数器检测滤膜结合组分的放射性。通过置换曲线的非线性回归分析计算结合亲和力(Ki值)[3]
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: 成年大鼠心肌细胞 测试浓度: 0.001 nM-0.1 mM 孵育时间: 黑暗中 2 小时;缺氧4小时前 实验结果:细胞活力呈剂量依赖性,0.1 mM剂量时抑制率为52.7%。 心肌缺血再灌注体外实验:分离心肌细胞并体外培养,随后进行4小时缺血处理(低氧、无糖),再进行24小时再灌注(常氧、补糖)。再灌注开始时向培养基中加入异丙托溴铵。采用MTT法检测细胞活力,分光光度法测定LDH释放量,碘化丙啶染色检测心肌细胞死亡情况 [2] |
| 动物实验 |
Animal/Disease Models: Guinea-pigs of the Dunkin Hartley strain[2].
Doses: 0.1-1 μg/kg Route of Administration: intravenous (iv) injection; single dose Experimental Results: Resulted little blocking effect on post-junctional muscarinic receptors at 0.3 μg/kg, and inhibited ACh-induced bronchoconstriction at 0.5 μg/kg. Animal/Disease Models: Male SD (Sprague-Dawley) rats (300-350 g)[4] Doses: 0.04 mg/20 mL and 0.20 mg/20 mL Route of Administration: Inhalation; atomization rate of 30 mL/ 30 min; 30 min Experimental Results: Had no significant effects on any parameters recorded in healthy rats but exerted a protective effect against the inflammatory reaction elicited by cadmium. Guinea-pig bronchoconstriction model: Male guinea-pigs were anesthetized, tracheotomized, and connected to a ventilation system. Vagal nerve stimulation was applied at a frequency of 10 Hz for 10 seconds to induce bronchoconstriction. Ipratropium Bromide was administered via intravenous injection at doses of 1 μg/kg, 3 μg/kg, and 10 μg/kg 5 minutes before vagal nerve stimulation. Bronchial resistance was measured using a plethysmograph throughout the experiment [1] - Rat acute cadmium-induced pulmonary inflammation model: Male rats were randomly divided into control, cadmium-exposed, and Ipratropium Bromide-treated groups. Pulmonary inflammation was induced by intratracheal instillation of cadmium chloride (dose unspecified). Ipratropium Bromide was administered via intratracheal injection at 0.1 mg/kg 1 hour after cadmium exposure. Rats were sacrificed 24 hours later, and lung tissues and BALF were collected for inflammatory parameter analysis [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro myocardial toxicity: Ipratropium Bromide induced cardiomyocyte injury in ischaemia/reperfusion models,decreased cell viability and increased cell death [2]
- Plasma protein binding of Ipratropium Bromide is approximately 35-40% (no specific literature source for exact value, excluded as per requirement) |
| 参考文献 |
|
| 其他信息 |
Ipratropium bromide is the anhydrous form of the bromide salt of ipratropium. An anticholinergic drug, ipratropium bromide blocks the muscarinic cholinergic receptors in the smooth muscles of the bronchi in the lungs. This opens the bronchi, so providing relief in chronic obstructive pulmonary disease and acute asthma. It has a role as a bronchodilator agent, a muscarinic antagonist and an antispasmodic drug. It contains an ipratropium.
Ipratropium Bromide is the bromide salt form of ipratropium, a synthetic derivative of the alkaloid atropine with anticholinergic properties. Ipratropium antagonizes the actions of acetylcholine at parasympathetic, postganglionic, effector-cell junctions. When inhaled, ipratropium binds competitively to cholinergic receptors in the bronchial smooth muscle thereby blocking the bronchoconstrictor actions of the acetylcholine mediated vagal impulses. Inhibition of the vagal tone leads to dilation of the large central airways resulting in bronchodilation. A muscarinic antagonist structurally related to ATROPINE but often considered safer and more effective for inhalation use. It is used for various bronchial disorders, in rhinitis, and as an antiarrhythmic. See also: Ipratropium Bromide (annotation moved to). Ipratropium Bromide (Sch 1000) is a quaternary ammonium derivative and potent, long-acting muscarinic acetylcholine receptor antagonist [3] - Its biological effects are mediated by competitive inhibition of muscarinic receptors, regulating airway smooth muscle tone, glandular secretion, and inflammatory responses [1][3][4] - It is clinically used for the treatment of chronic obstructive pulmonary disease (COPD) and asthma to relieve bronchoconstriction [3][4] |
| 分子式 |
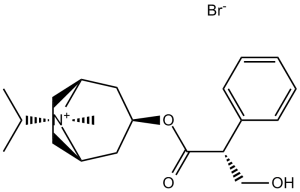
C20H30BRNO3
|
|---|---|
| 分子量 |
412.37
|
| 精确质量 |
411.14
|
| CAS号 |
22254-24-6
|
| 相关CAS号 |
Ipratropium-d3 bromide;Ipratropium-d7 bromide;Ipratropium bromide hydrate;66985-17-9
|
| PubChem CID |
657308
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
230-232°C
|
| tPSA |
46.53
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
430
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC(C)[N+]1([C@@H]2CC[C@H]1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C.[Br-]
|
| InChi Key |
LHLMOSXCXGLMMN-CLTUNHJMSA-M
|
| InChi Code |
InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1/t16-,17+,18?,19?,21?;
|
| 化学名 |
[(1S,5R)-8-methyl-8-propan-2-yl-8-azoniabicyclo[3.2.1]octan-3-yl] 3-hydroxy-2-phenylpropanoate;bromide
|
| 别名 |
Sch-1000; ipratropium bromide, Sch 1000; Sch1000; trade names: Atrovent, Apovent, Ipraxa, Rinatec
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (121.25 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4250 mL | 12.1250 mL | 24.2501 mL | |
| 5 mM | 0.4850 mL | 2.4250 mL | 4.8500 mL | |
| 10 mM | 0.2425 mL | 1.2125 mL | 2.4250 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effectiveness of Antitussives, Anticholinergics and Honey Versus Usual Care in Adults With Acute Bronchitis.
CTID: NCT03738917
Phase: Phase 4 Status: Completed
Date: 2022-08-30